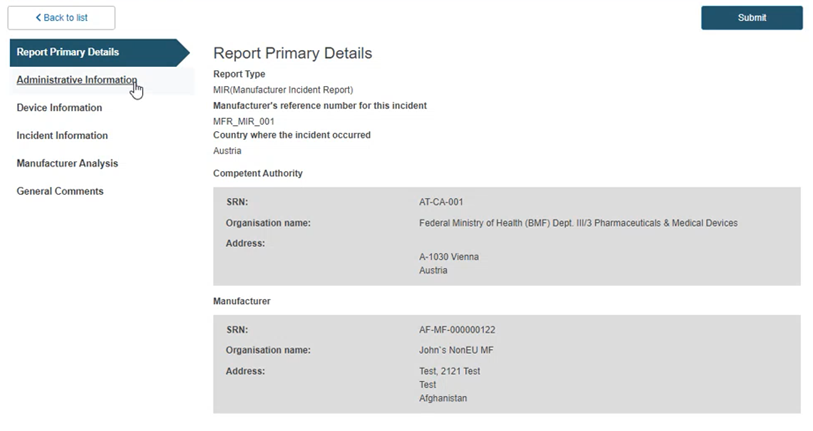
Administrative information
Click on the Administrative Information section from the menu on the left:
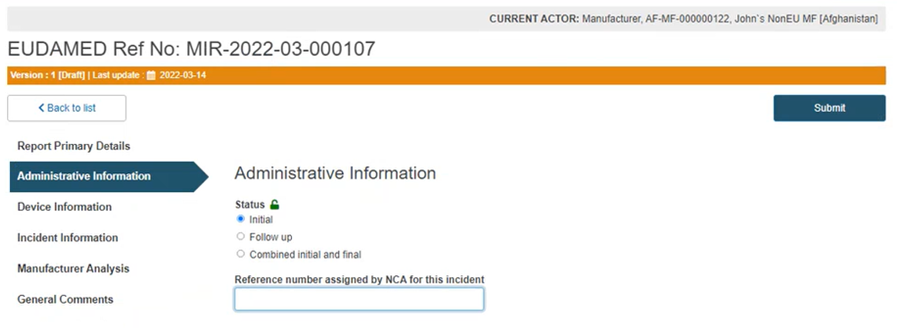
Choose the status of the MIR and provide the reference number assigned by the National Competent Authority in the field below:
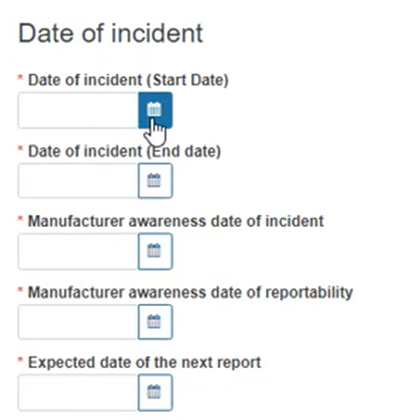
Provide all the relevant dates relating to the incident:
Note
The Expected date of the next report refers to cases of a submission of a MIR in state Initial or Follow-up, otherwise it will not be visible to fill in.
Select the classification of the incident:

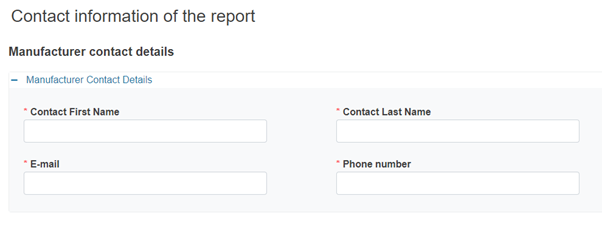
Provide the contact details of the report in the corresponding sections:
Note
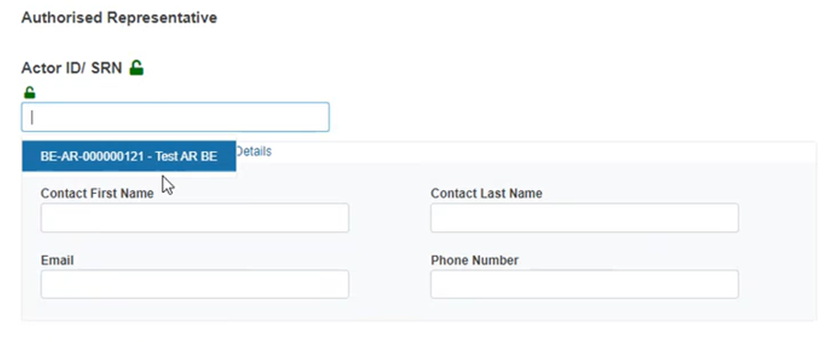
Non-EU manufacturers must provide the Actor ID / SRN of the concerned Authorised Representative (regardless of the AR’s mandate status):
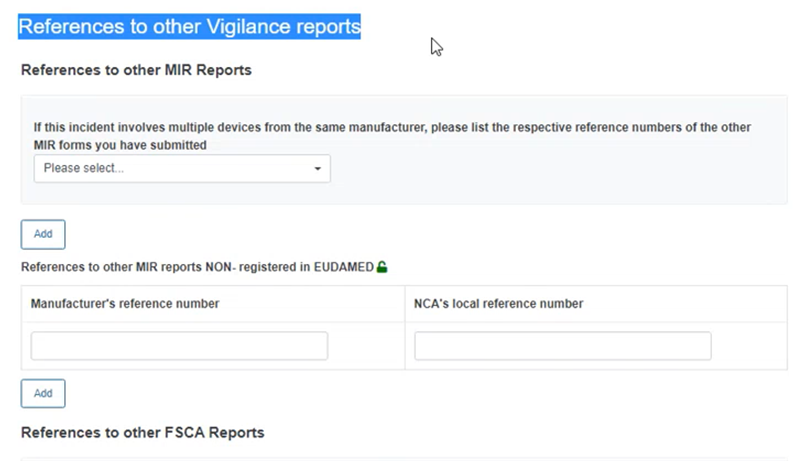
If you wish to link this MIR to other MIRs, please provide the relevant references:
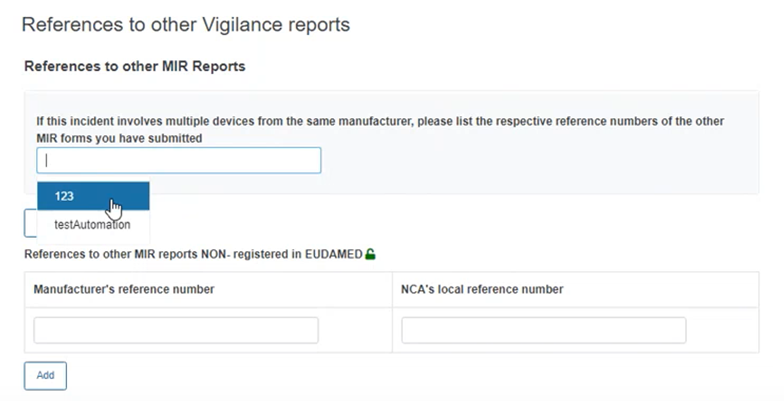
Insert the Manufacturer’s reference number of the concerned report and select it from the list:
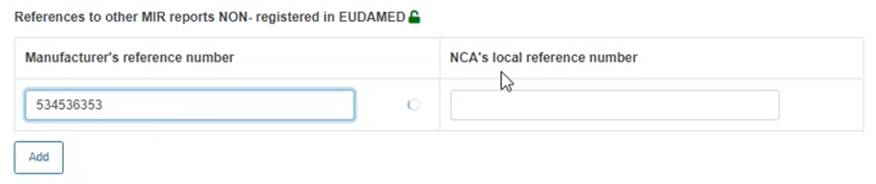
In case the report is not yet registered in EUDAMED, use the field for non-registered reports to type the manufacturer reference of the concerned report:
If applicable, provide additional reference to PSR reports or PMCF/PMPF investigations:

If the incident occurred within a PMCF/PMPF investigation, provide the EUDAMED ID of the relevant investigation:
