Device information
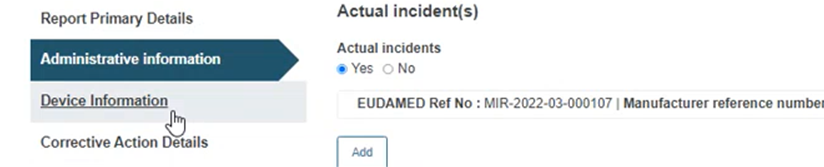
Click on the Device Information section from the menu on the left:
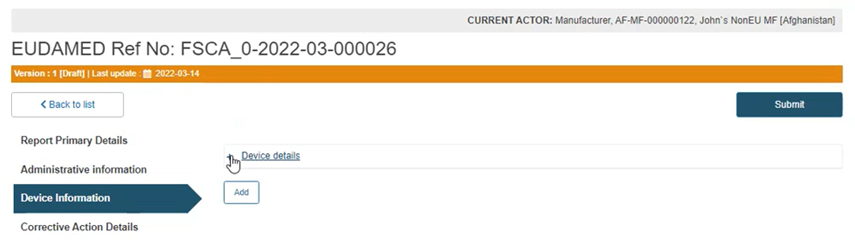
Click on the plus sign next to Device details to access the fields to be filled:
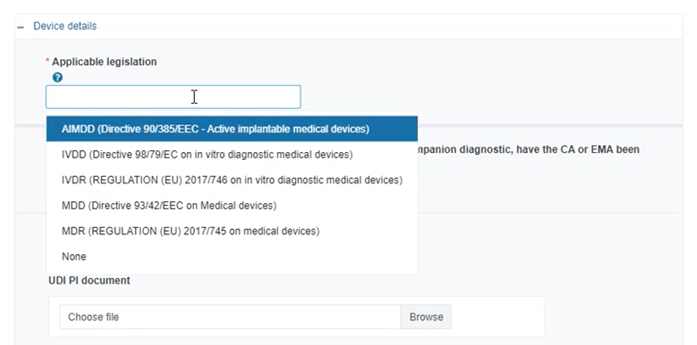
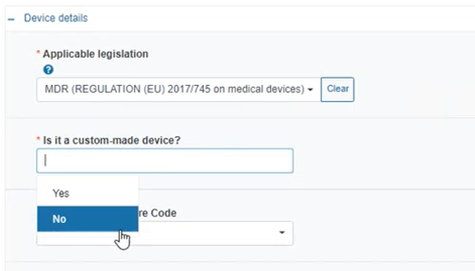
Select the applicable legislation:
Specify if the device is custom-made:
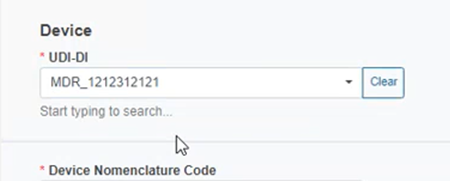
Provide the UDI-DI:
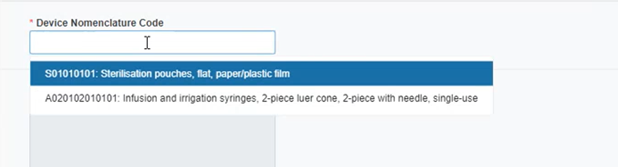
Select the EMDN nomenclature code:
The system will auto-fill the Nomenclature text field:
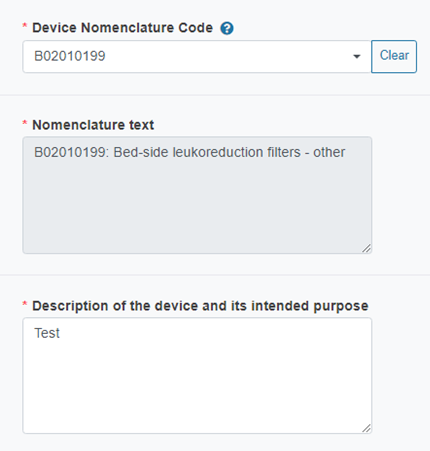
Describe the device and its intended purpose:

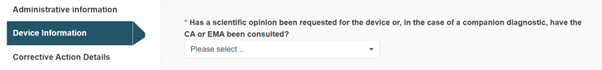
Answer the next question with Yes or No:
Important
The mandatory field regarding a scientific opinion above will only appear for MDR and IVDR legislations.
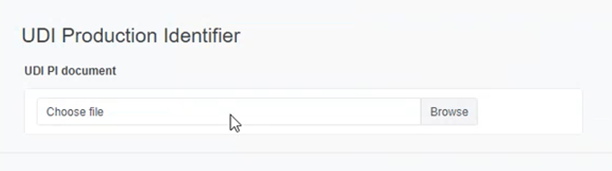
Click on Browse to upload a UDI PI Document in PDF format:
Click on the plus sign next to Certificate Identification and complete the appearing fields:
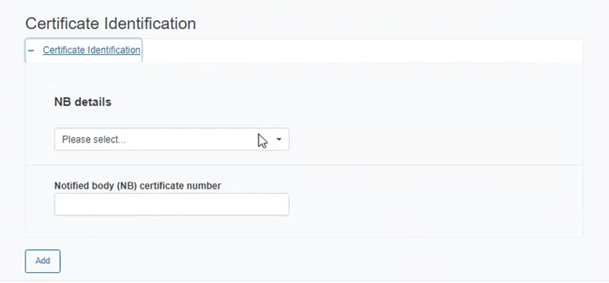
Select the Notified Body details from the drop-down list and insert the NB certificate number for the device.
Select the appropriate Device date and fill in the year and month:
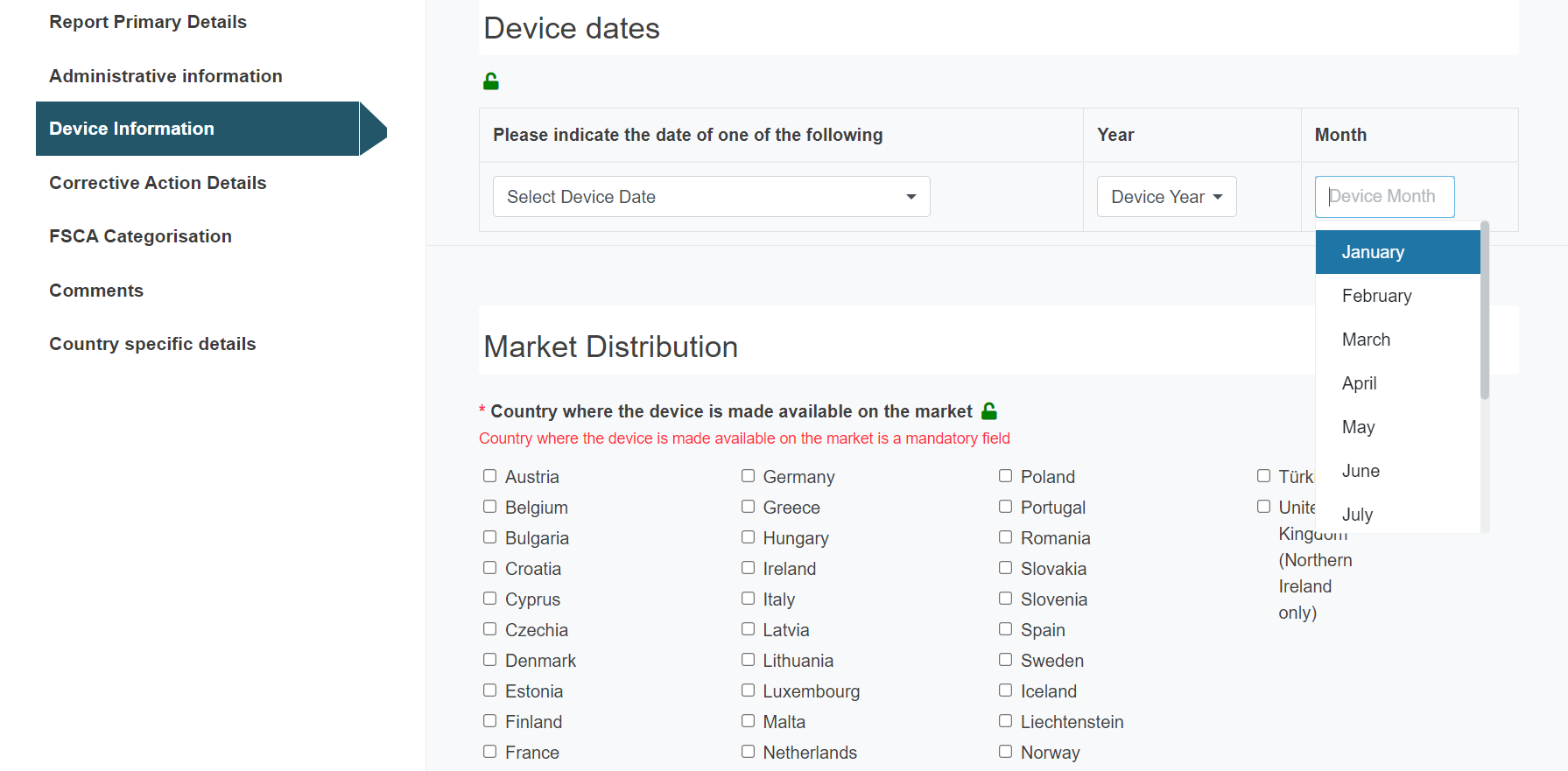
Tick the countries where the device is made available on the market:
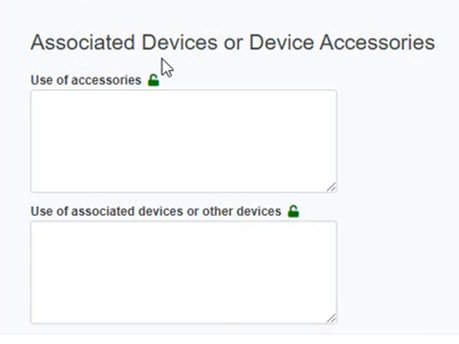
Provide information for the Associated Devices and Device Accessories section: