Verifying non-EU manufacturer registrations
[Specific to AR Verifier]
Although the national competent authorities are responsible for validating a non-EU manufacturer's registration request, an additional preliminary step is required from the Authorised Representative: they must verify the information provided by the manufacturer, as well as the summary mandate details.
To verify a non-EU manufacturer registration request
Log in to EUDAMED with a Verifier, LAA or LUA profile (for the Authorised Representative). If there are outstanding registration requests from non-EU manufacturers, you will see the following link in your dashboard:
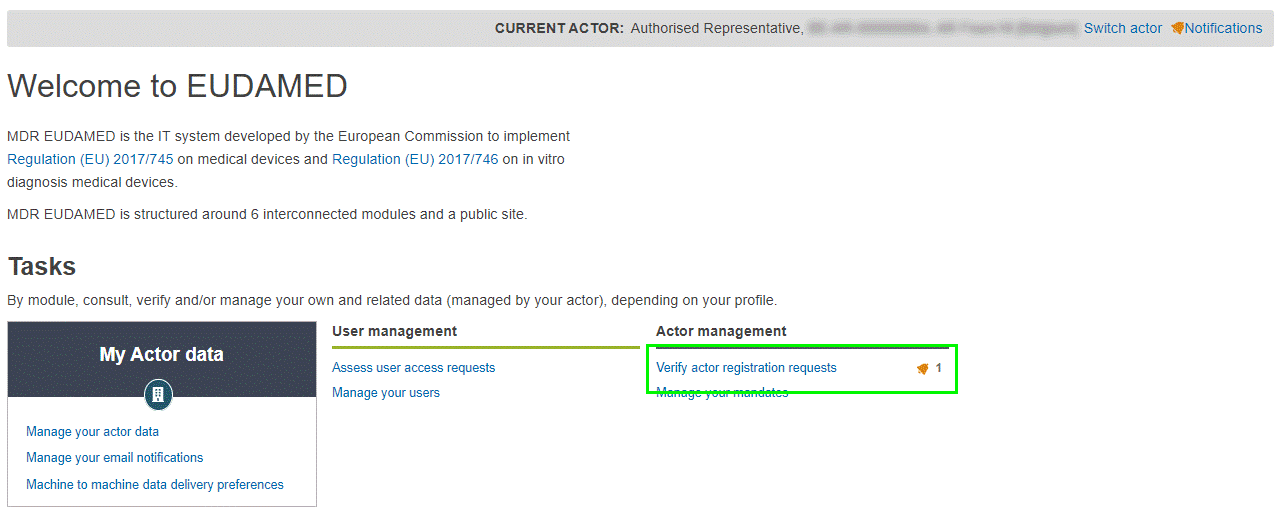
Click Verify actor registration requests in the Actor management section of the dashboard. The Actor registration management page lists all pending non-EU manufacturer registration requests waiting to be verified by your actor (if any):
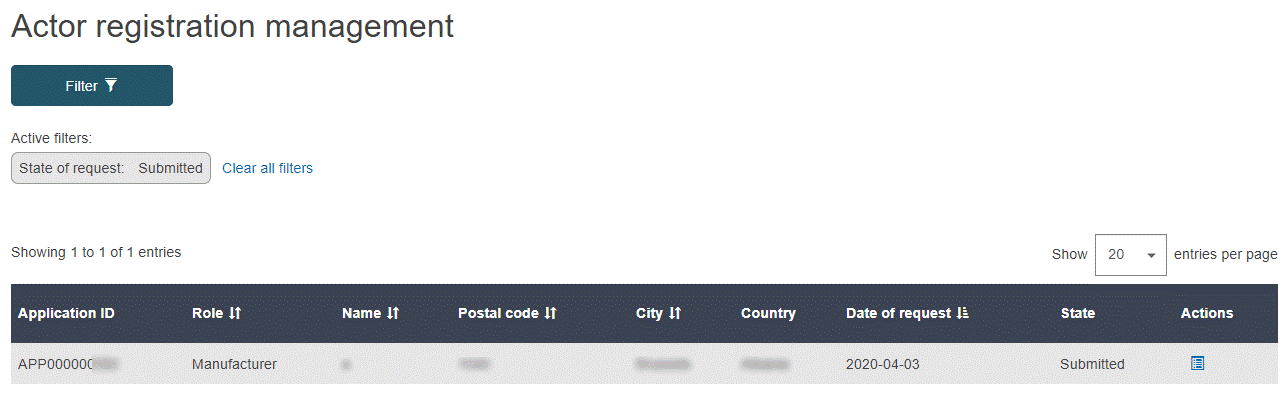
Click
 Assess in the Actions column to review and assess the desired registration request in the list. All details of the selected registration request, as submitted by the non-EU manufacturer, are displayed.
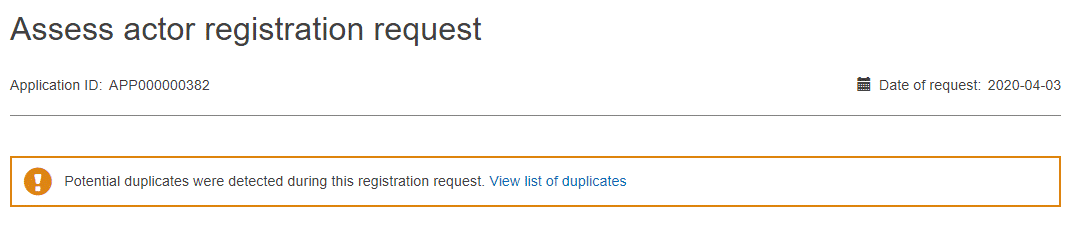
Assess in the Actions column to review and assess the desired registration request in the list. All details of the selected registration request, as submitted by the non-EU manufacturer, are displayed.Review the information. A warning message will flag possible duplicates, so select the correct registration request from the list available:
In the Assessment section, slide the toggle left to Approved or right to Not approved.
If you choose to approve the request, you may enter additional notes.
If you choose not to approve the request, you will be prompted to the Justify your decision with one of the following reasons:
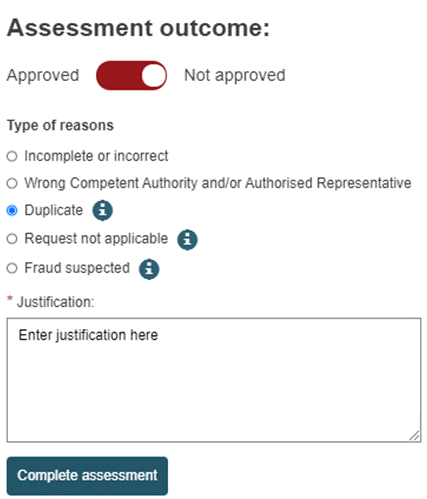
Incomplete or incorrect
Some information that you deem important is either missing or looks incorrect. The requesting user will have a chance to correct or complete the request following your instructions and re-submit the request.
Wrong Competent Authority and/or Authorised Representative
You are not the correct Authorised Representative for this request. The requesting user will have a chance to select another and re-submit.
Duplicate
Informs the requesting user that this actor already exists.
Request not applicable
The request will be rejected and cannot be re-submitted.
Fraud Suspected:
The requesting user will be informed that registration was not successful. The reason and justification provided will not be shared with the requesting user but will be visible for competent authorities.
Click Complete assessment at the bottom of the page, and then Confirm in the subsequent confirmation pop-up. You are informed that the non-EU manufacturer's registration request has been assessed.
If approved, its status now changes to Verified. This means the request has been submitted to the relevant Competent Authority for validation:
