Device information
Click on Device information from the menu on the left:
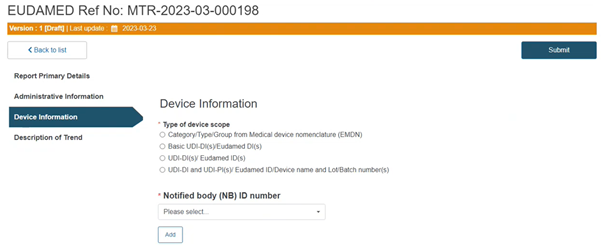
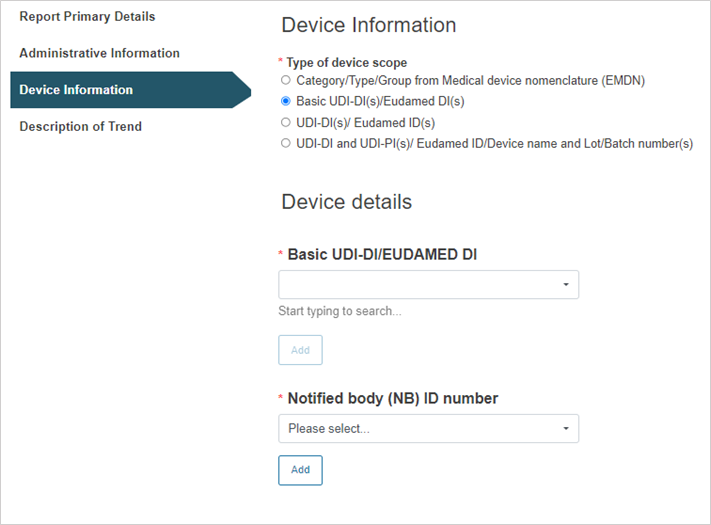
Select the type of the device scope:
Depending on the device scope type selected, the process will vary slightly, as shown in detail in each of the four options below.
Steps for Category/Type/Group from Medical device nomenclature (EMDN):
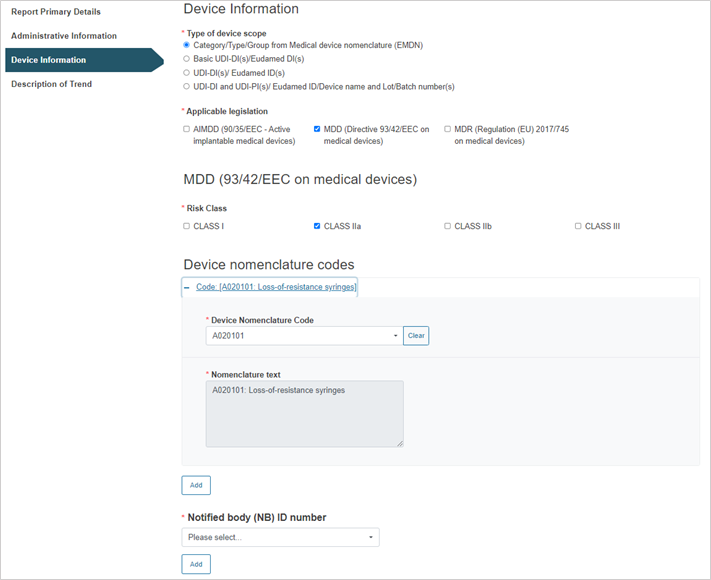
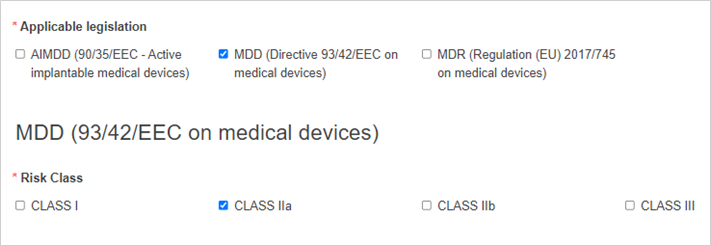
Select the applicable legislation and risk class:
Enter the device nomenclature code:
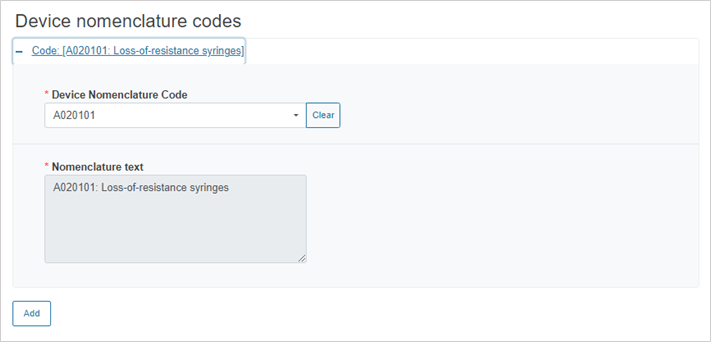
To add more nomenclature codes, click on the Add button.
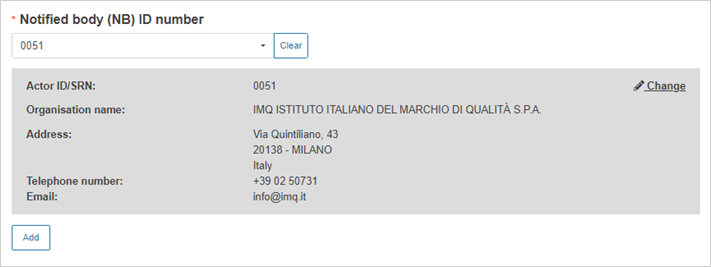
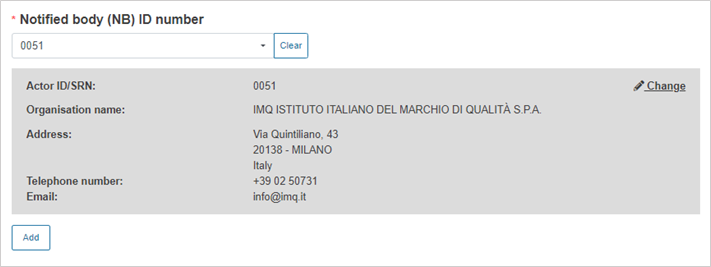
Select the Notified Body ID number:
Steps for Basic UDI-DI(s)/EUDAMED DI(s):
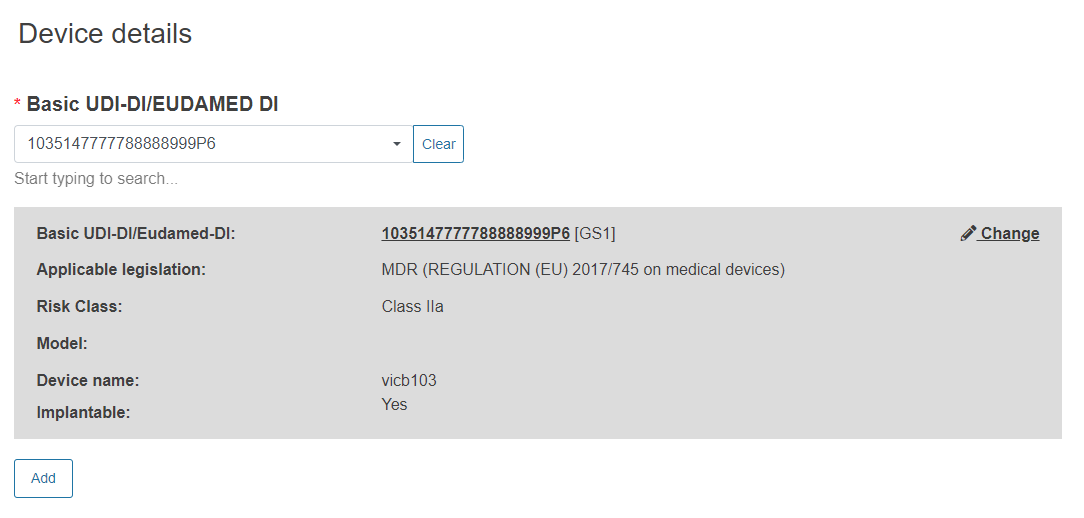
Enter the Basic UDI-DI/EUDAMED DI:
Select the Notified Body ID number:
Steps for UDI-DI(s)/EUDAMED ID(s):
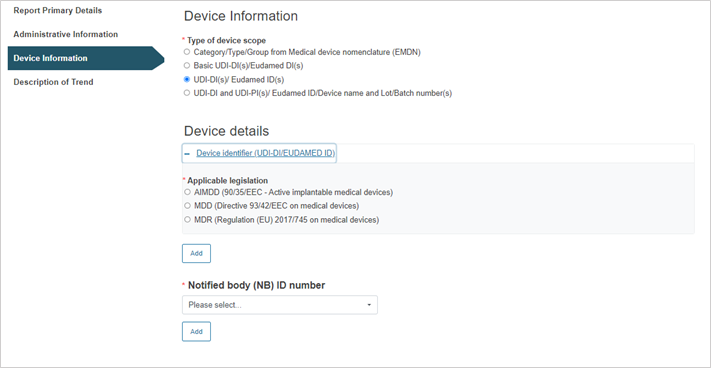
Click on the plus sign next to Device identifier to select the applicable legislation.
If the legislation selected is AIMDD, MDD or IVDD, the system will display the following mandatory question:
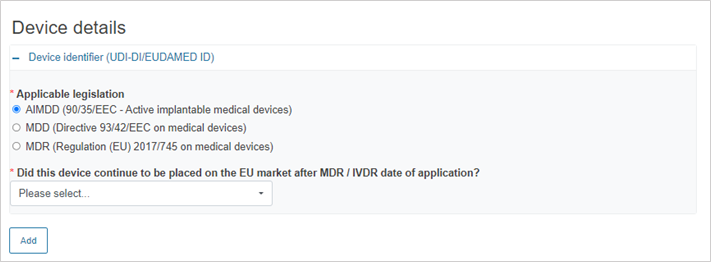
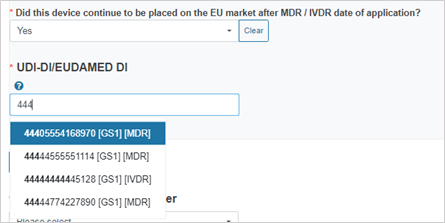
If you answer Yes, provide the device identifier:
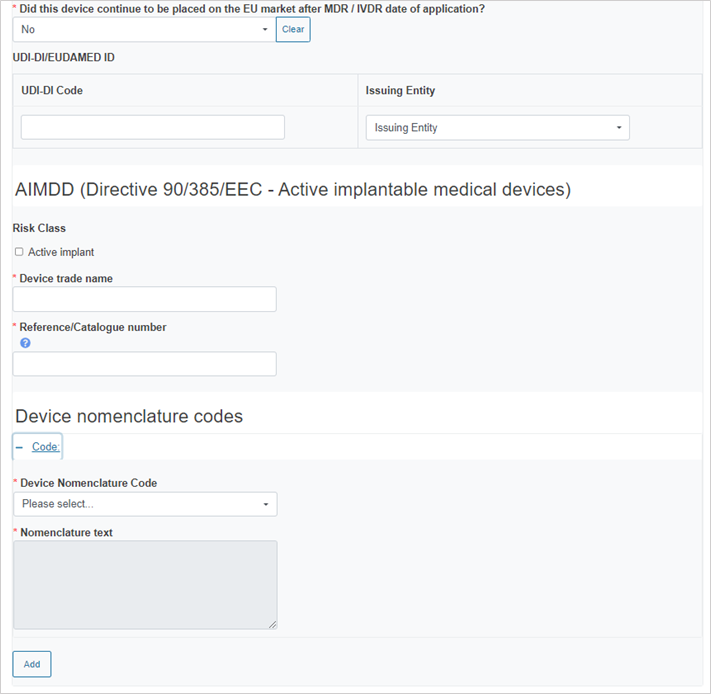
If you answer No, provide the device information:
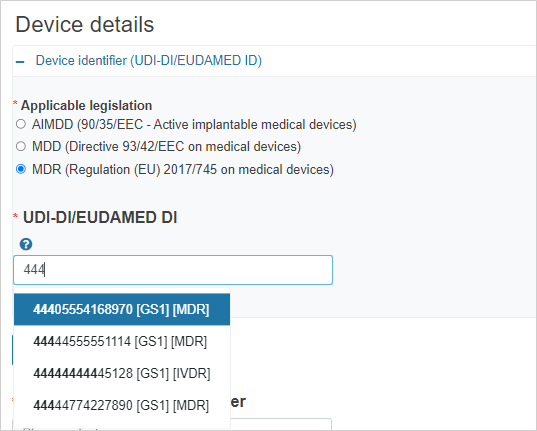
If the legislation selected is MDR/IVDR, enter the UDI-DI/EUDAMED ID:
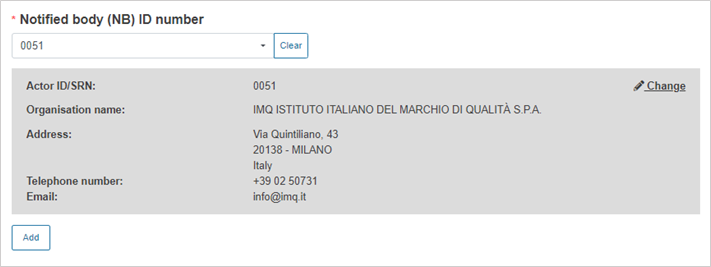
Select the Notified Body ID number:
Steps for UDI-DI and UDI-PI(s) / EUDAMED ID(s) / Device name and Lot/Batch Number(s):
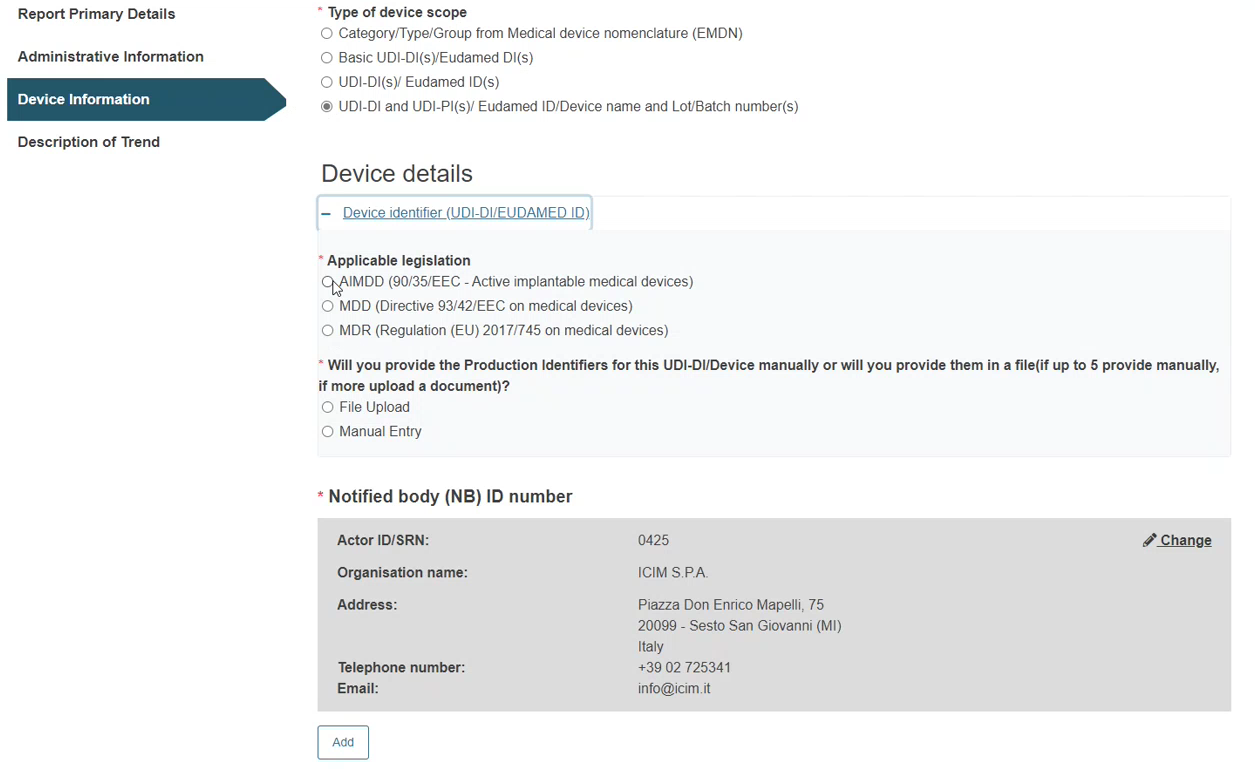
Click on the plus sign next to Device identifier to select the applicable legislation.
If the legislation selected is AIMDD, MDD or IVDD the system will display the following mandatory question:

If you answer Yes, provide the device identifier:

If you answer No, provide the device information:

If the legislation selected is MDR/IVDR enter the UDI-DI/EUDAMED ID:

Enter the Production Identifiers either manually (up to 5 entries) or click Browse to upload a file:
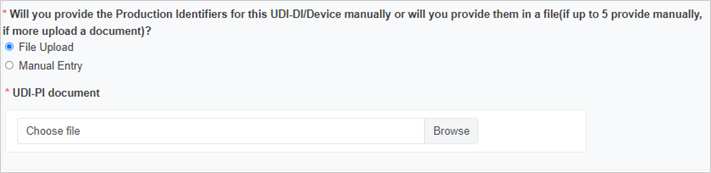
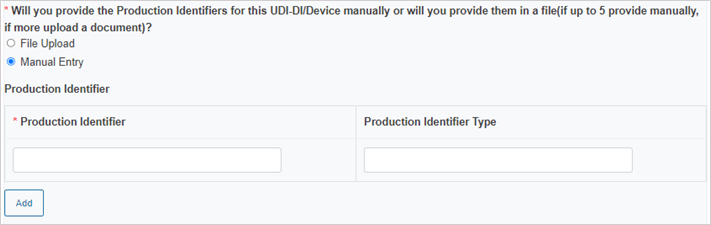
Select the Notified Body ID number:
