Introduction
Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnosis medical devices introduce an EU identification system for medical devices based on a Unique Device Identifier (UDI).
The UDI-DI/Device module of EUDAMED is used for the manufacturers to provide their UDIs/Devices information and to make it available to everyone.
Warning
EUDAMED does not contain all constraints defined in the MDR/IVDR, guidance and good practices, and therefore, it is not because something is possible in EUDAMED that it is necessarily allowed.
 VIDEO: What is a UDI?
VIDEO: What is a UDI?
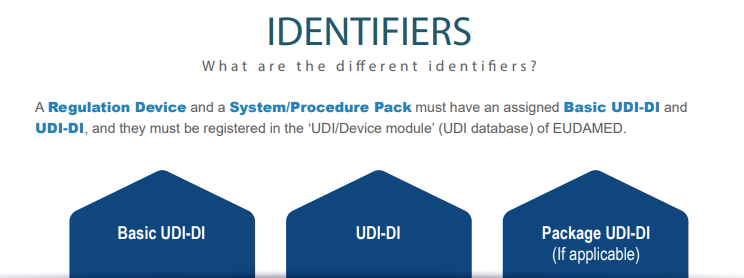
 INFOGRAPHIC: Basic UDI-DI/UDI-ID concept
INFOGRAPHIC: Basic UDI-DI/UDI-ID concept