UDI-DI characteristics
Specify clinical size for the UDI-DI if applicable and choose the dimension and the precision values in the drop-down lists below:
Note
When the selected Clinical size type has the option Other, users will be required to enter the Description of the Clinical size type and the language of description. The same applies for Measure unit.
In case both the Clinical size and Measure unit have the option Other, the description for the two fields needs to be provided in the same languages.

You must provide one of the following precision types:
Range – requires minimum and maximum values and the measure unit
Text – requires free text entry
Value – requires the size and the measuring unit
You may add several clinical sizes by adding different types of dimensions, but only one dimension for a given type.
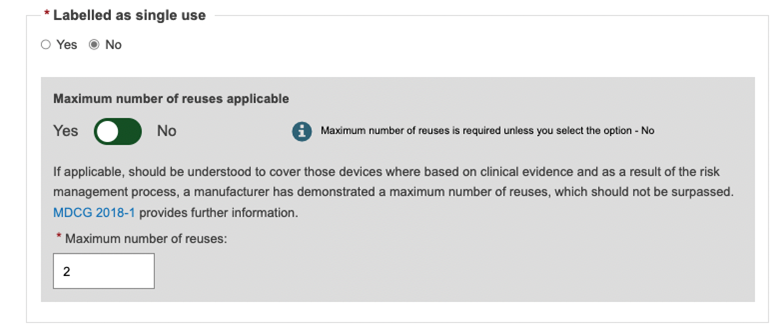
Specify if the device is labelled as single use.
When the device is not labelled single use you must provide the number of reuses if applicable:
If the Maximum number of reuses is not applicable, then the device is considered as non-single use Device and it does not have a maximum number of reuses (infinite number of reuses)
If value provided is >=1, the device is considered as a non-single use Device having a limited number of reuses (the value provided)
Select Yes or No for each of the options below:

Note
Containing latex is only for MDR, not applicable for IVDR.
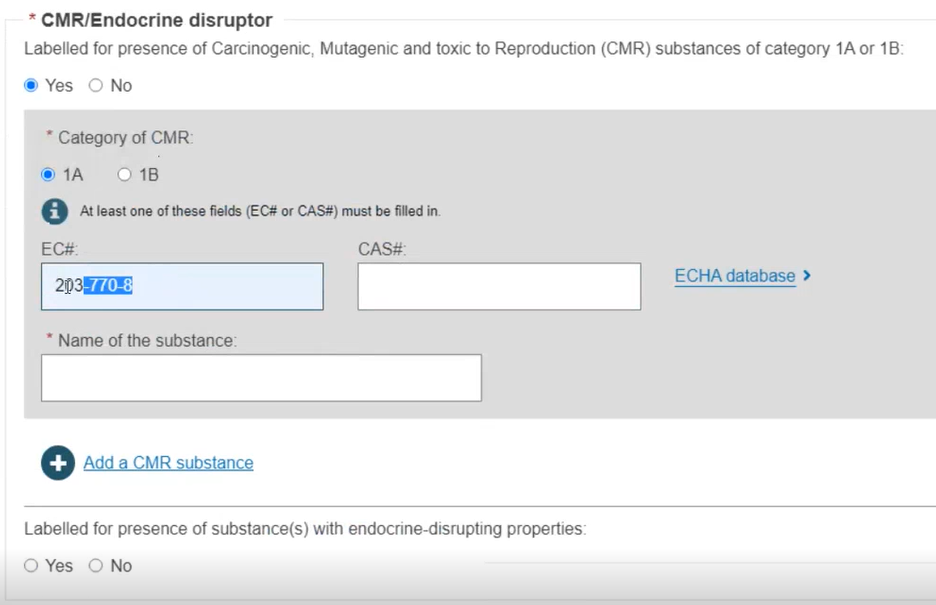
For MDR enter the CMR and/or Endocrine disruptor substances if applicable. When specifying CMR and/or Endocrine substances you may provide the EC# or CAS#. If you do provide them, only the Name of substance is required (the language is no longer required):
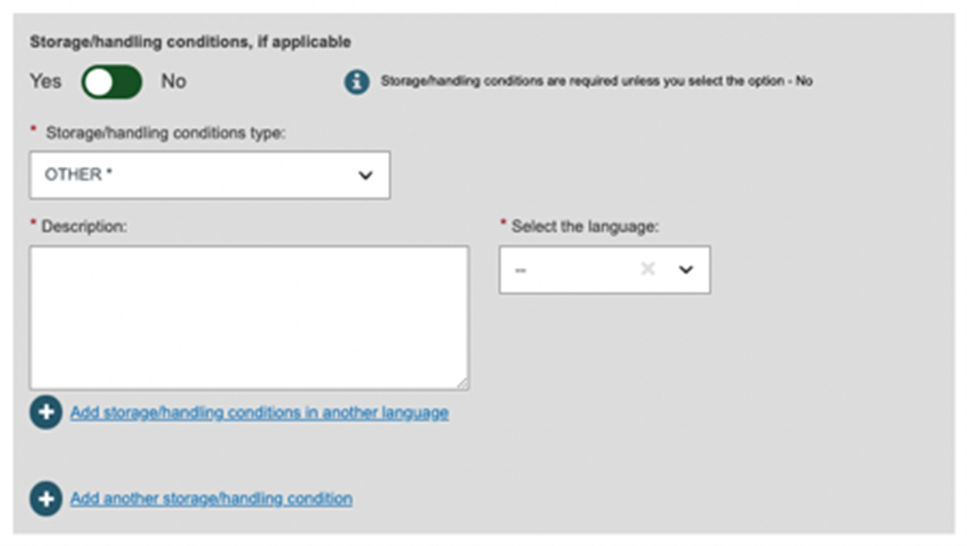
Fill in the Storage/handling conditions section:
Note
For Storage/handling conditions type Other, users must enter the Description of the Storage/handling condition type and the description's language.
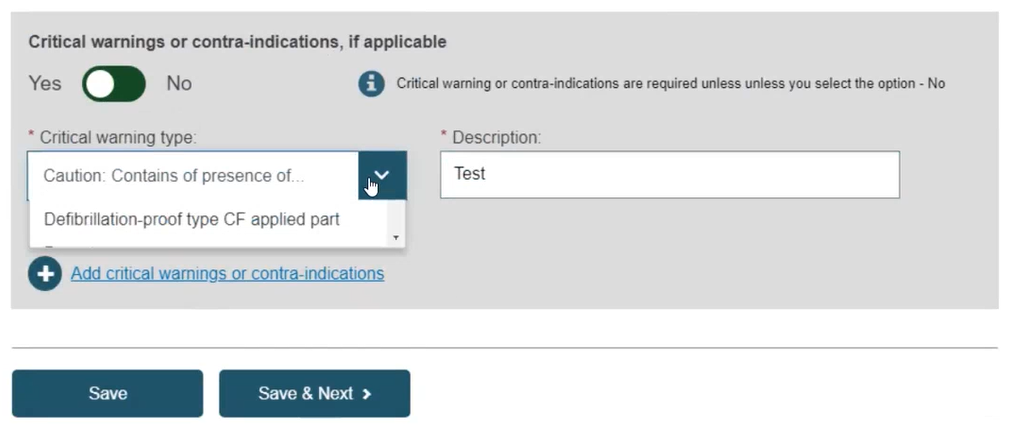
Fill in Critical warnings or contra-indications, and click Save or Save & Next:
Note
For Critical warning or contra-indications type Other, users must enter the Description of the Critical warning or contra-indications type and the description's language.