UDI-DI identification information
 VIDEO: UDI carrier and display formats
VIDEO: UDI carrier and display formats
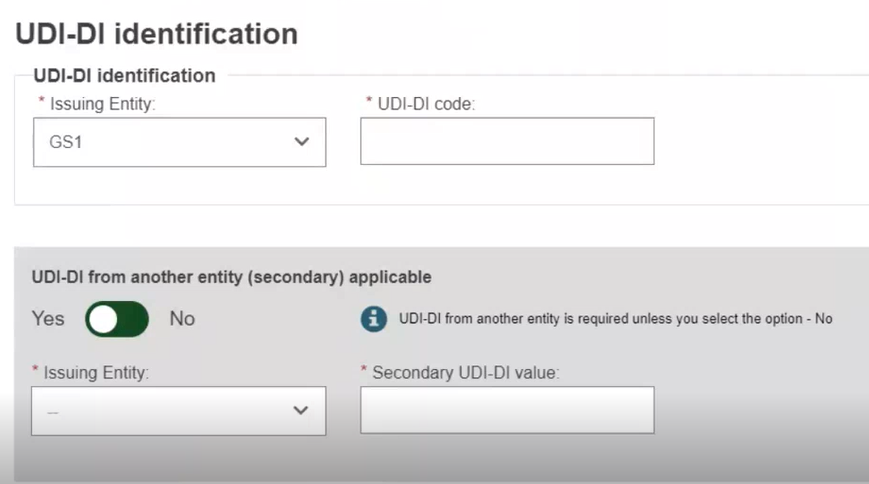
Select the Issuing Entity from the drop-down list and enter the UDI-DI code.
Important
The UDI-DI code you enter must be unique. If it already exists in EUDAMED, you will not be able to Save.
Exception: the same UDI-DI can be used for a Legacy Device and its Regulation Device equivalent.
If the same UDI-DI code was already provided for a Legacy Device (i.e. Applicable Legislation MDD, AIMDD or IVDD), you will be prompted that a link will be created between the two devices (the Regulation and the Legacy Device) on the condition there is no conflict between some of the Basic UDI-DI properties and the related legacy device EUDAMED DI properties. In case of conflict, the system will prevent you from using the same UDI-DI.
Note
In the case of a GS1 Issuing Entity, the UDI-DI code you enter must be a 14-digit code including the check digit that will be used by EUDAMED to validate the UDI-DI code. If your GS1 UDI-DI (GTIN code) is less than 14 digits (check digit included), when populating EUDAMED field, please add leading zero(s) until you reach 14 digits.
For example:
000000nnnnnnnn (GTIN-8)
00nnnnnnnnnnnn (GTIN-12)
0nnnnnnnnnnnnn (GTIN-13)
If applicable, enter the Secondary UDI-DI from a different Issuing Entity to the UDI-DI:
Enter the EMDN code and click on Find, and select the correct one from the list:

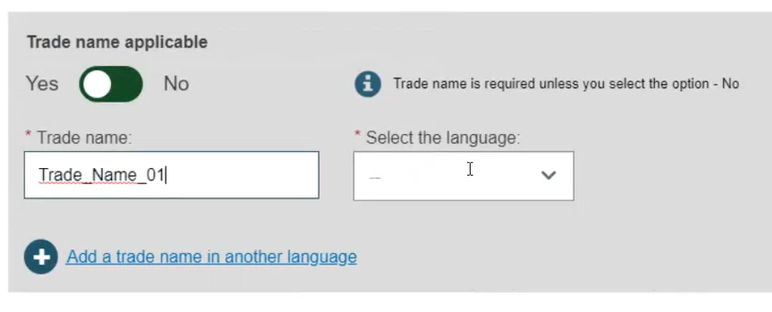
If applicable, enter the trade name (as specified on the device label) and select its related language (select All languages if not language dependent):
Enter the Reference/Catalogue number:
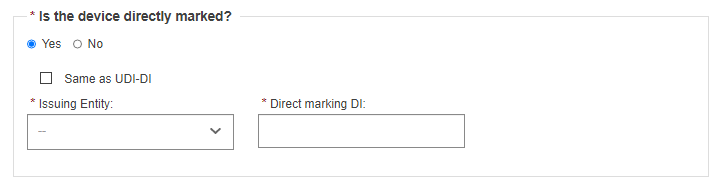
Specify whether the device is directly marked or not:
If the device is directly marked, you must either indicate it is the same as the UDI-DI or enter the UDI-DI and issuing entity of the Direct marking DI.
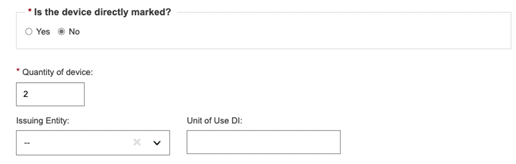
If the device is not directly marked and the base quantity of the device is greater than one, you may enter the Unit of Use DI and its issuing entity:
The same Unit of Use DI can be used for different UDI-DIs in case the same device has different root packaging (each one having a different UDI-DI).
If the base quantity is less than two, then no unit of use Di is provided:
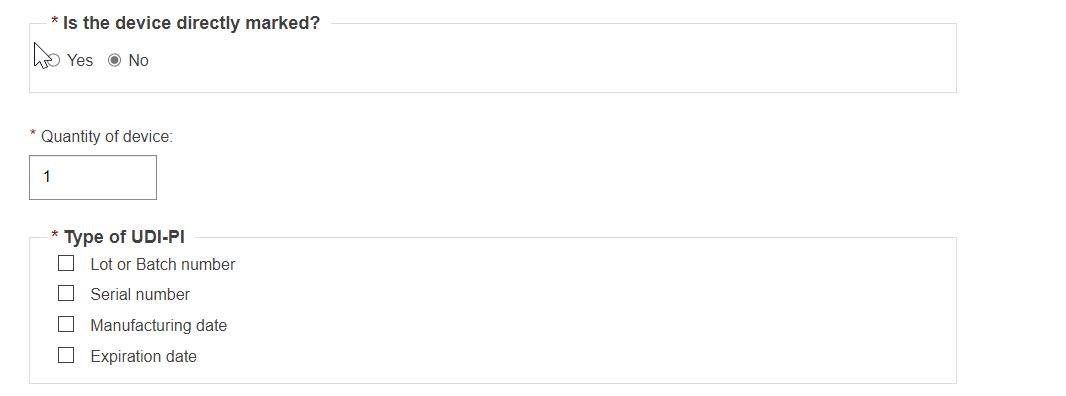
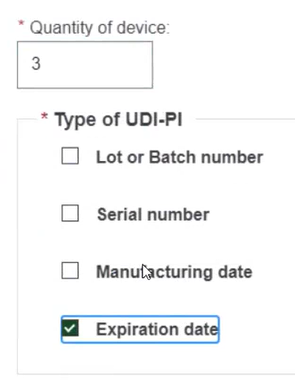
Select the Type of UDI-PI:
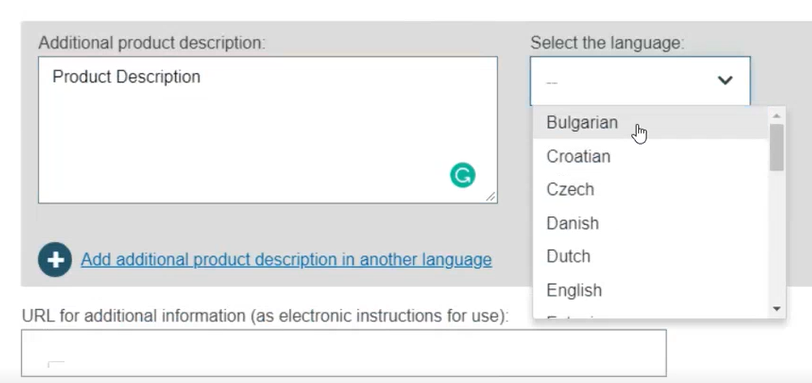
Enter any additional information you think important to specify about the device, select the language in which the additional information is provided and enter a URL (web address) if you have one for additional information online:
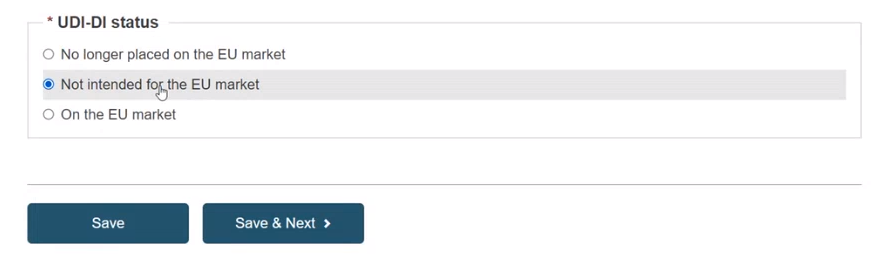
Specify the UDI-DI status in selecting whether it is On the EU market, Not intended for the EU market or No longer placed on the EU market and click on Save or Save & Next: