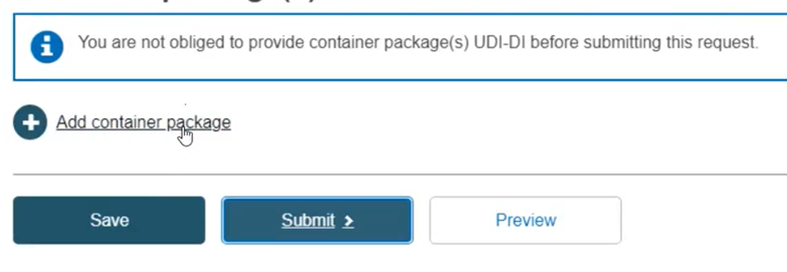
Container package details
 VIDEO: UDI and Systems and Procedure Packs
VIDEO: UDI and Systems and Procedure Packs
Click on Add container package when there is a higher packaging level for the root UDI-DI:
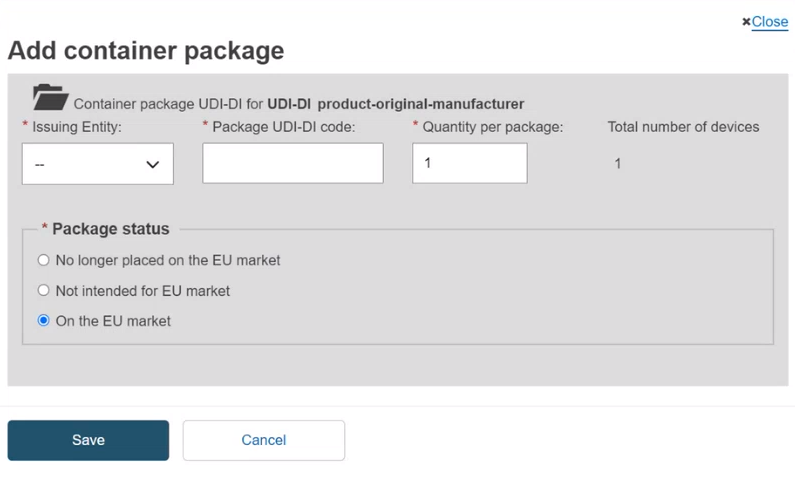
A unique UDI-DI must be assigned to each package level. You add a higher container package to the root UDI-DI if there is no container package UDI-DI yet, or to the selected UDI-DI (you can add as many levels and as many container packages per level as you have). Add the Issuing Entity, Package UDI-DI code and the Quantity per package, select the Package status and click on Save:
Note
If the UDI-DI already exists in EUDAMED, the system will prevent you from saving.
Note
If the status of the device for this container package is either No longer placed on the EU Market or Not intended for the EU Market, the Package status options are greyed out and any container package added to this device will automatically have the same Package status as the device.
Select the generated information and click on Submit:
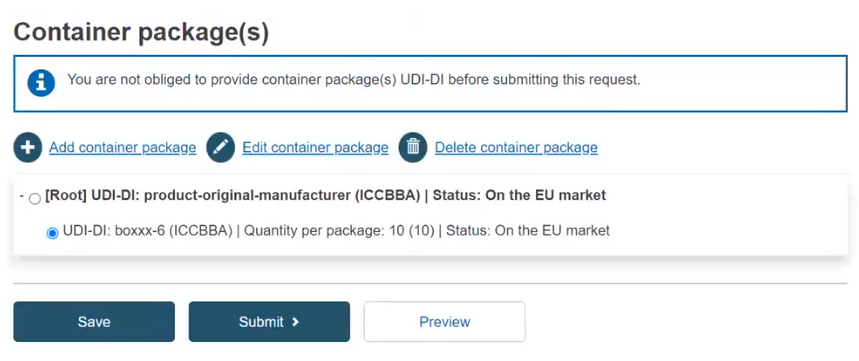
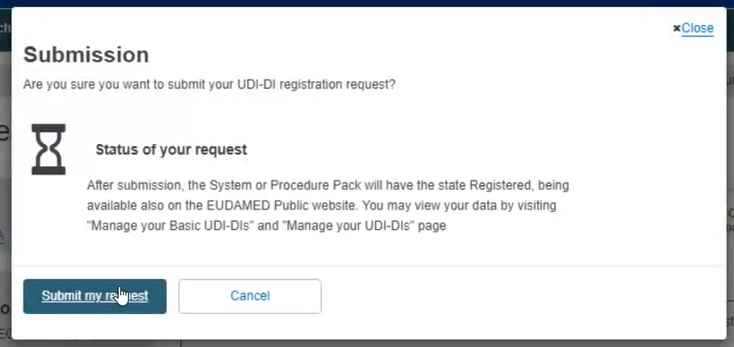
As a final step, a pop-up window will appear, asking you to confirm that you are ready to submit your registration request. If so, click on Submit my Request:
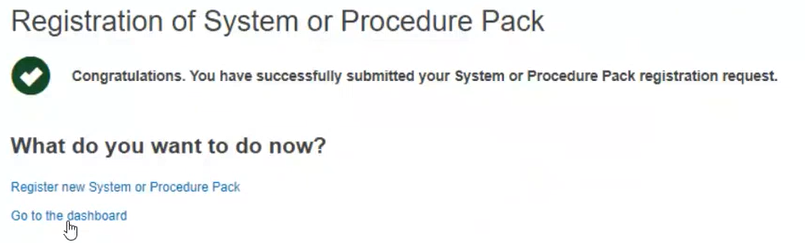
Upon submission, you will see a message that you have successfully submitted an SPP registration request: