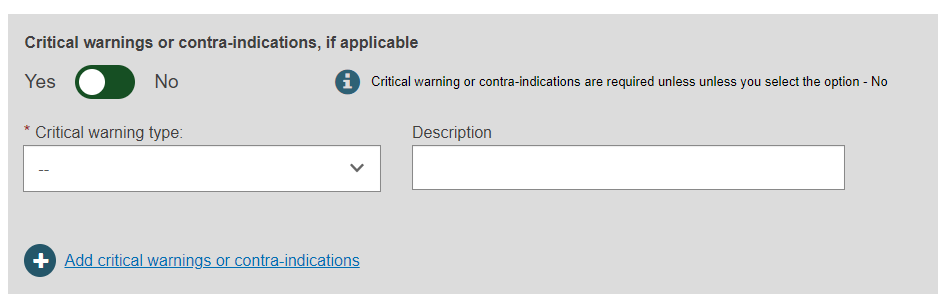
Device characteristics
Select Yes or No for the first three options, then select Yes or No whether if Storage/handling conditions are applicable:
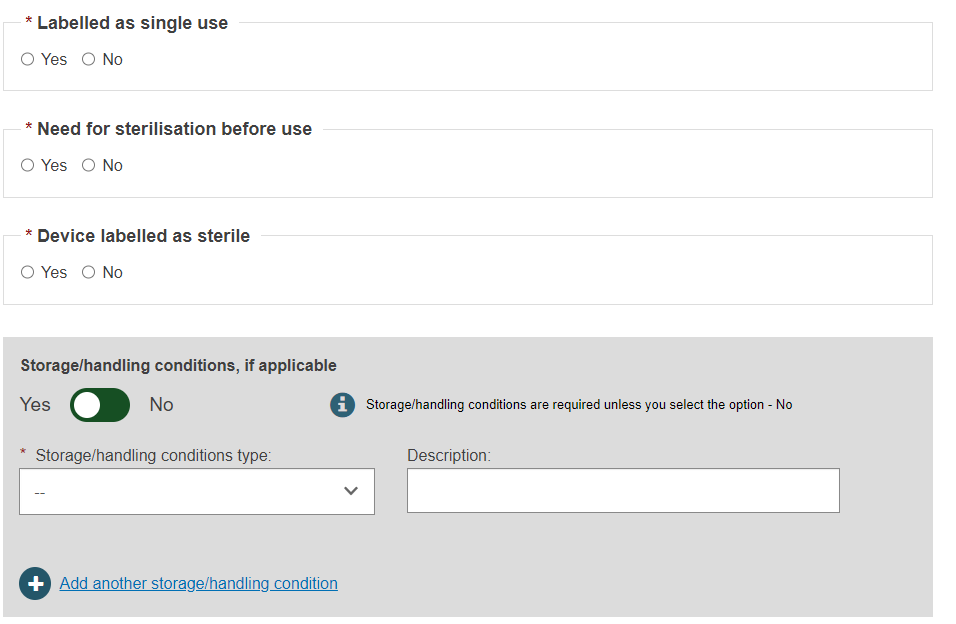
If applicable, provide the correct values by selecting from the options provided and enter a description:
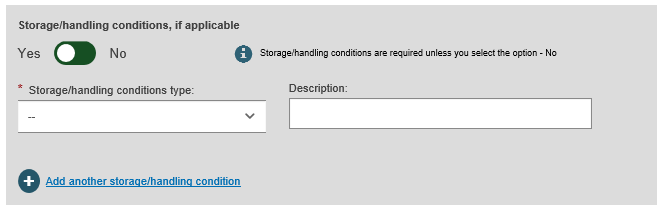
Select Yes or No for Critical warnings or contra-indications and if Yes, enter the type and description. After completing, click on Save or Save & Next: