Provision of core data
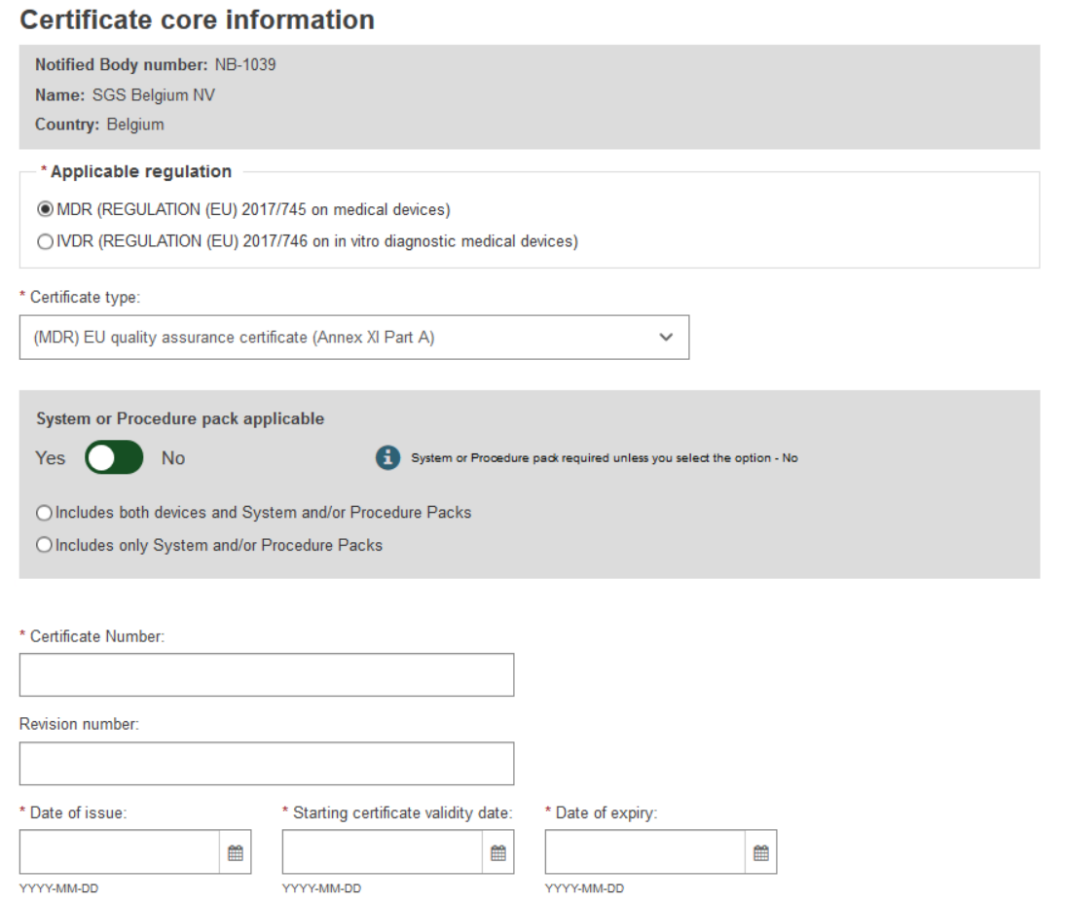
Select the applicable regulation, and the certificate type. The type of the certificate will change depending on the regulation, MDR or IVDR.
Note
In this scenario we will choose a Quality certificate type.
Select Yes or No whether the certificate is for a system or procedure pack.
Enter the Certificate Number and a Revision number if applicable.
Enter the Date of issue, the Starting certificate validity date and the date of expiry:
Note
Manufacturer information
Enter the Actor ID/SRN or name of the manufacturer or the system/procedure pack producer, click Find and select from the list displayed.
Click Save & Next.