Basic UDI-DI main information
On the EUDAMED dashboard, click on Register a New System Procedure Pack:
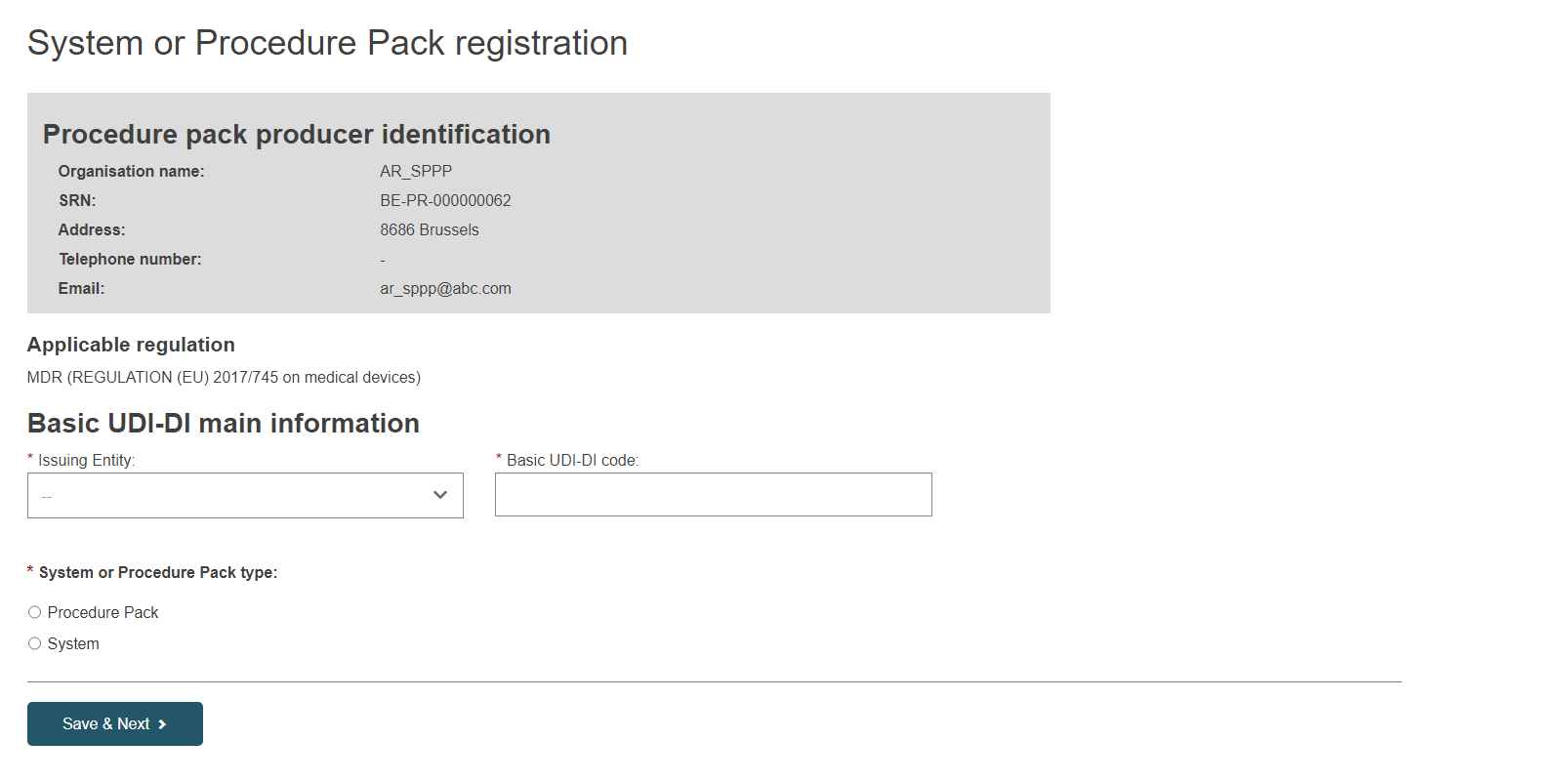
On the next page, specify the Issuing Entity and the Basic UDI-DI code:
Note
Only the applicable legislation MDR (Regulation (EU) 2017/745 on medical devices) is possible for system and procedure packs (selected by default).
Important
EUDAMED will validate the Basic UDI-DI code you insert based on the specific format provided by each Issuing Entity. Please ensure that you enter the correct code with the check digits.
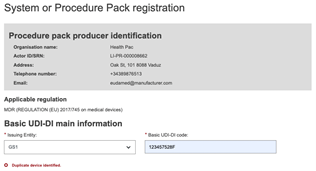
If the Basic UDI-DI code already exists in EUDAMED, the system will prevent you from saving – a Basic UDI-DI must be unique:
Choose if you are registering a system or procedure pack and click on Save & Next to save your registration as a draft and move on to the next steps:
