Provision of certificate details
Select the correct options:

Note
When you have defined the scope of the certificate with a device group and/or device having risk class I that has the property Placed on the market in sterile condition, then the system will set Yes for the Devices in sterile condition question within the Special Device Type within the scope.
Enter the conditions and limitations if there are any. If none, toggle to No:
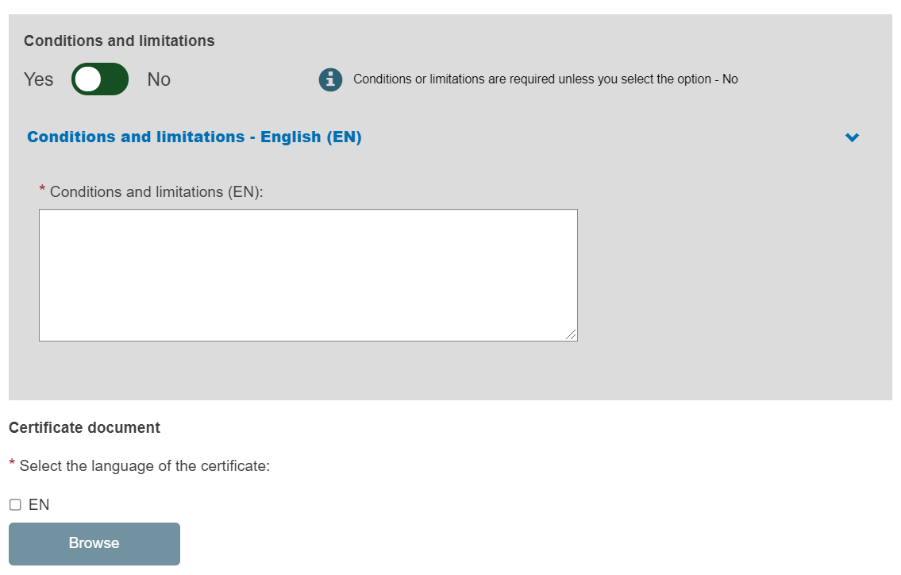
Select the languages in which the electronic version of the certificate is issued. You may upload more than one electronic document if it covers different languages, and you may upload several documents at once.
Click on either Save or Save & Next.