Device group and device
A device group or a device is mandatory to provide for Quality-type certificates.
Click + Add a device group:
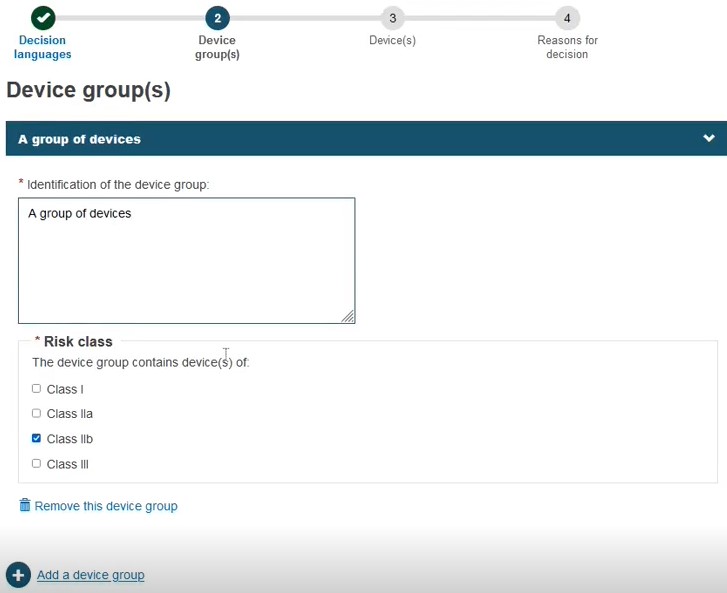
Provide either a Name, Reference/Catalogue number or Basic UDI-DI. Note that these options also apply when registering a Refused/withdrawn application for a Quality-type certificate:

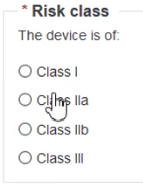
Choose the Risk class:
Depending on the risk class, choose Yes or No whether the device is custom-made or not:
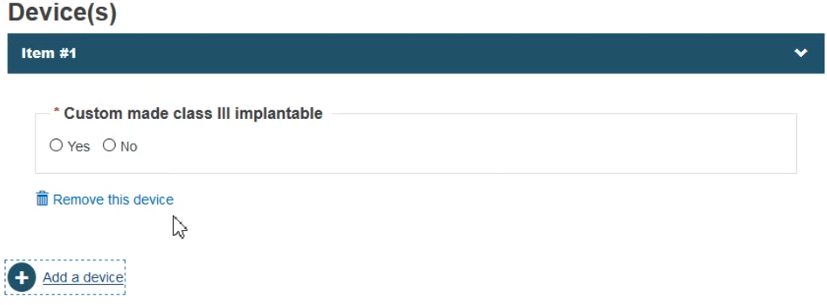
Click Save & Next.
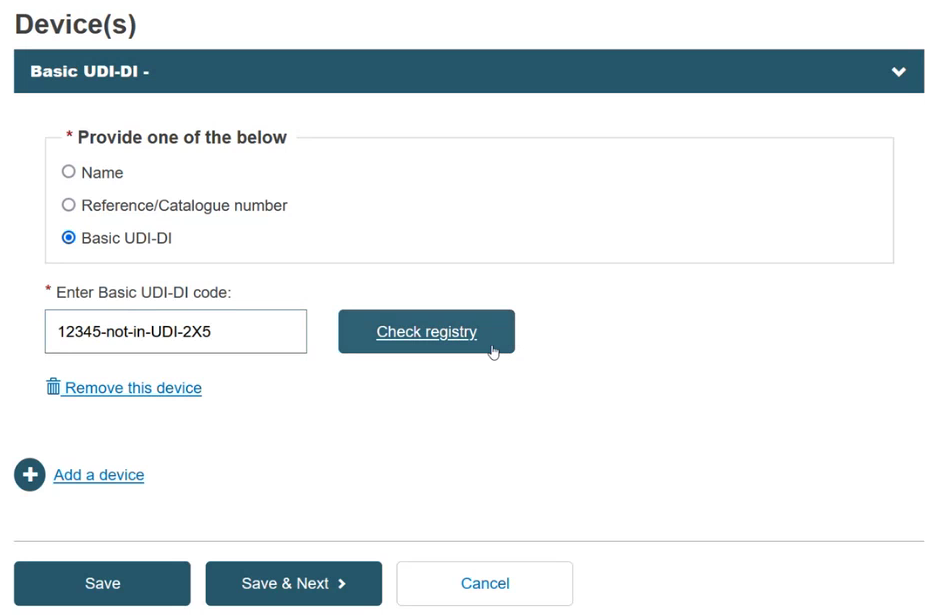
Add a device and its Basic UDI-DI by clicking + Add a device:
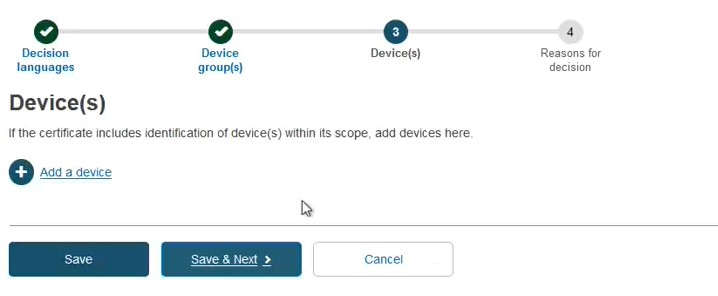
Provide either a Name, Reference/Catalogue number or Basic UDI-DI:

If you select the Basic UDI-DI, the Enter Basic UDI-DI code field is displayed.
Enter the Basic UDI-DI code and click Check Registry:
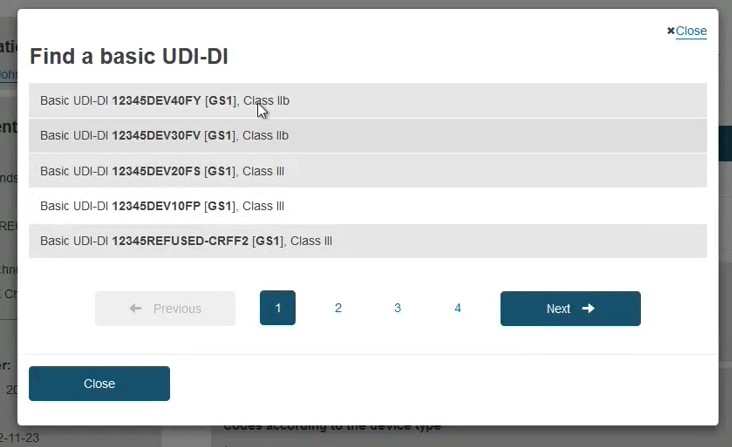
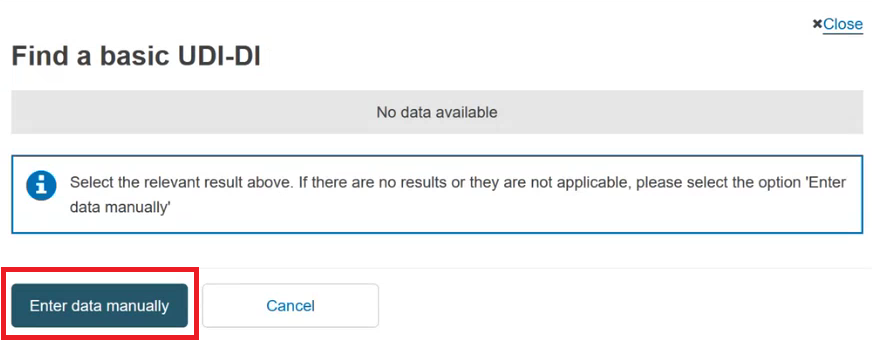
In the pop-up window either select the device from the list (if the device is already registered in EUDAMED) or click the Enter data manually button (if the device is not yet registered in EUDAMED) to add the Basic UDI-DI manually:
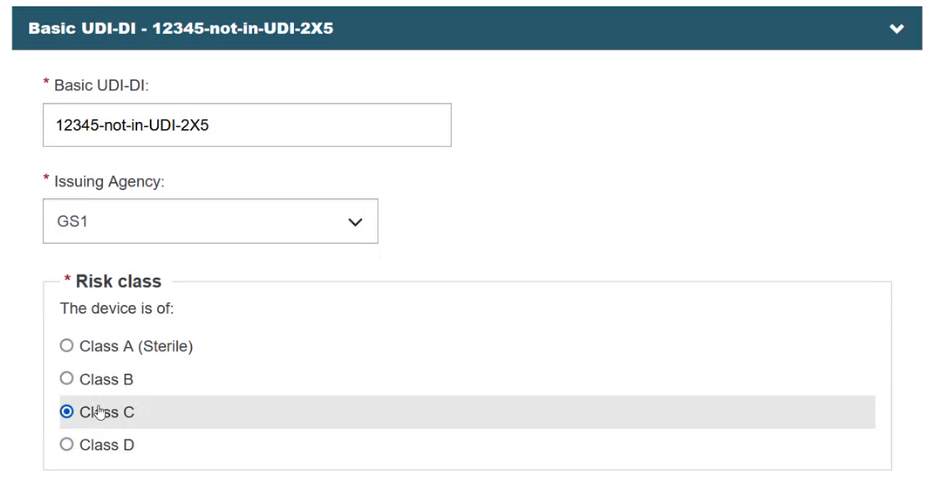
After clicking on the Enter data manually button, a new screen appears. Provide the Issuing Agency and the risk class of the device:
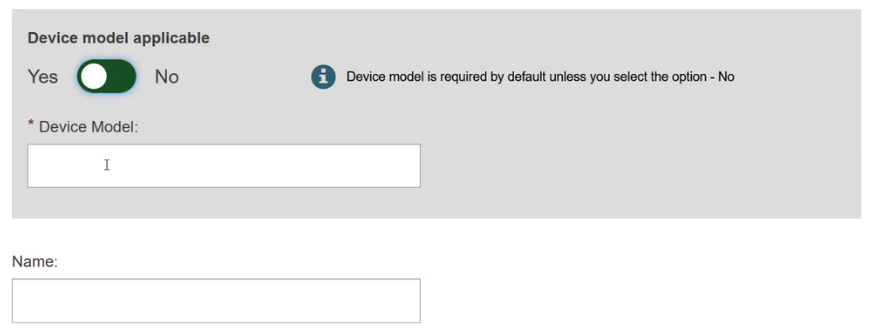
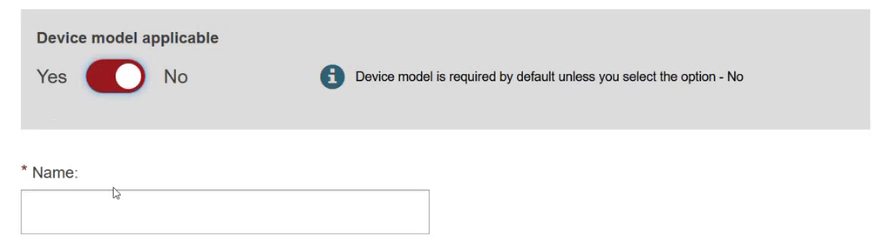
Specify if the device model is applicable or not:
Click Save & Next.