Device information
Select Yes or No if the device was designed and manufactured by another legal or natural person.
If Yes, there are two different ways to find the Product original manufacturer of the device:
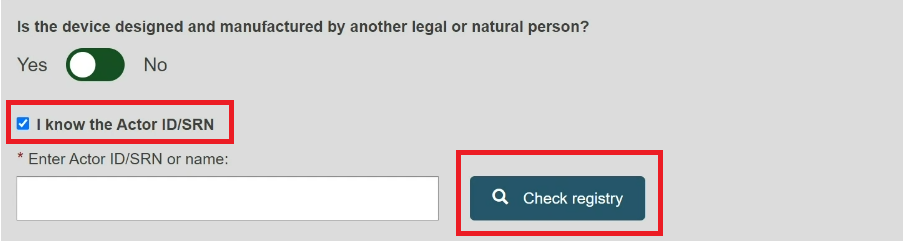
Check the box I know the Actor ID/SRN, enter the Actor ID/SRN or name of the Product original manufacturer of the device and click Check registry:
Note
Please ensure to check the box I know the Actor ID/SRN in order to search for an existing registered Manufacturer Actor either by SRN or by name.
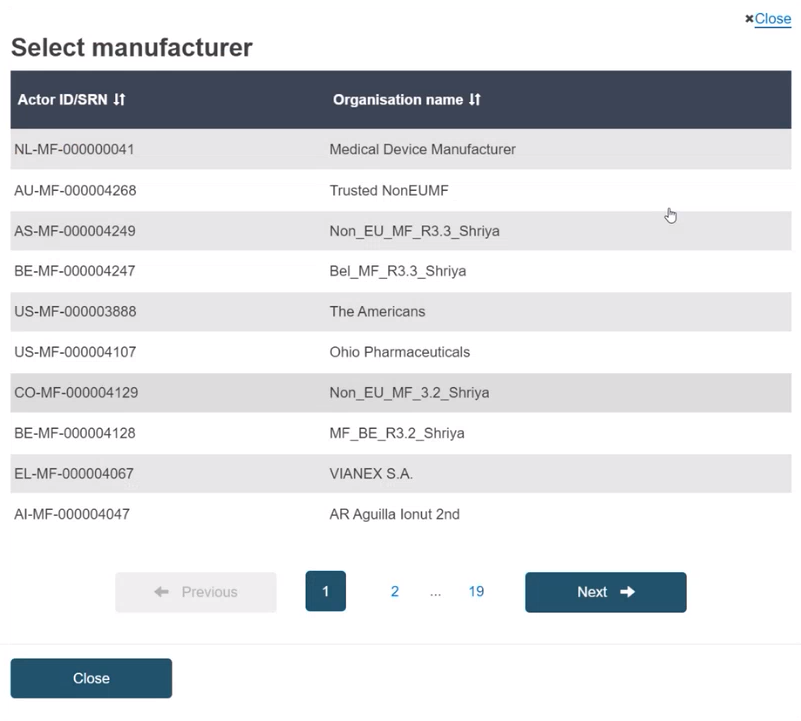
Select the Actor from the list:
Enter the name of the Product original manufacturer organisation name and click on Check registry:
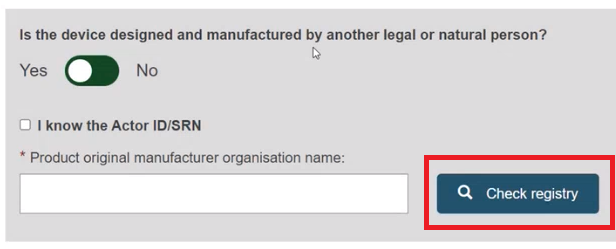
Select the Organisation name from the list:
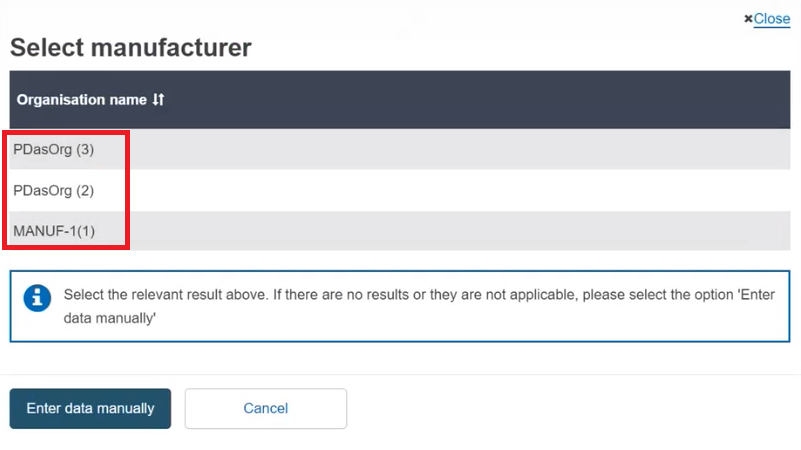
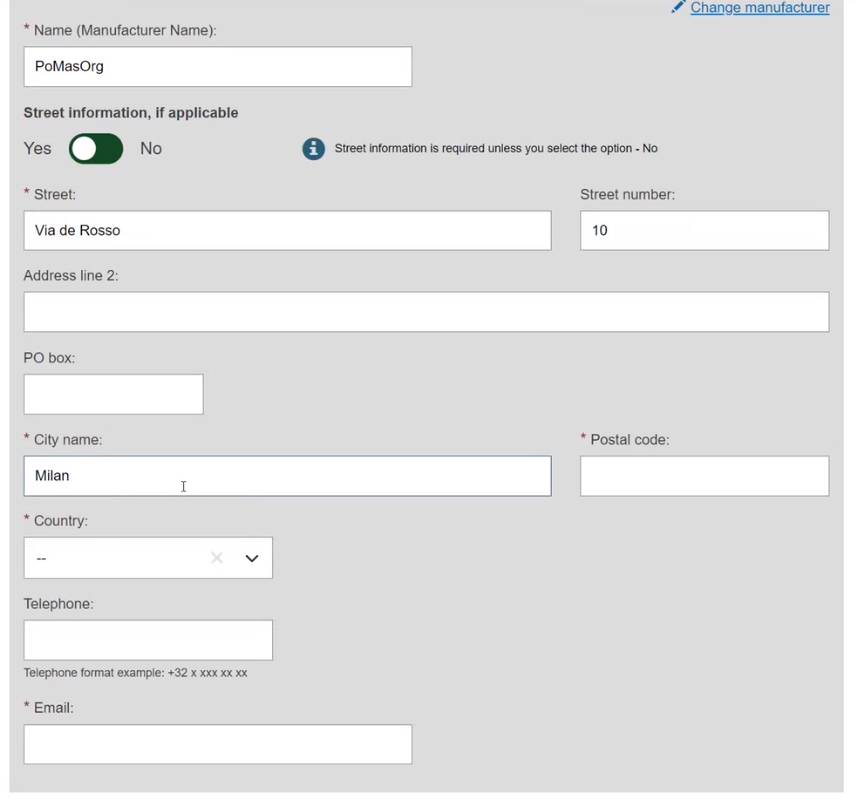
If the Organisation name is not in the list, click on Enter data manually and fill in the required fields with the details on the Product original manufacturer of the device:
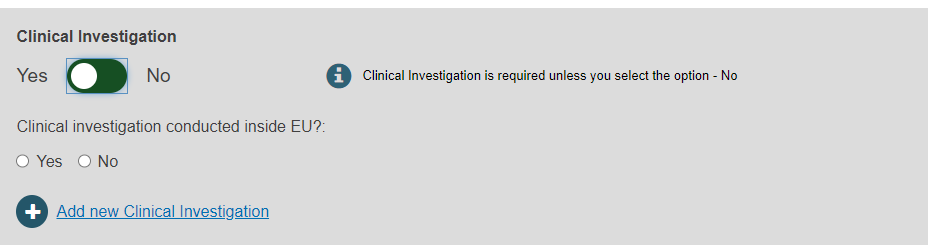
Select Yes or No to provide the Clinical Investigation reference(s):
Select Yes or No for the three following options on Tissues and cells:
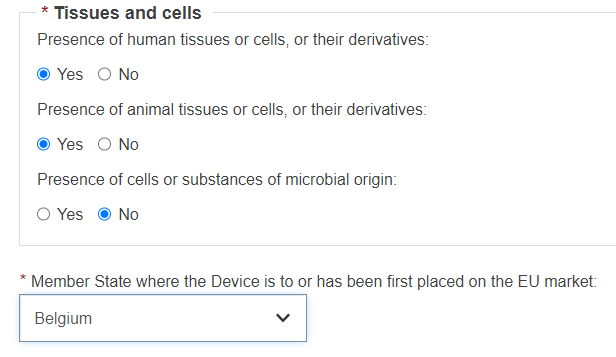
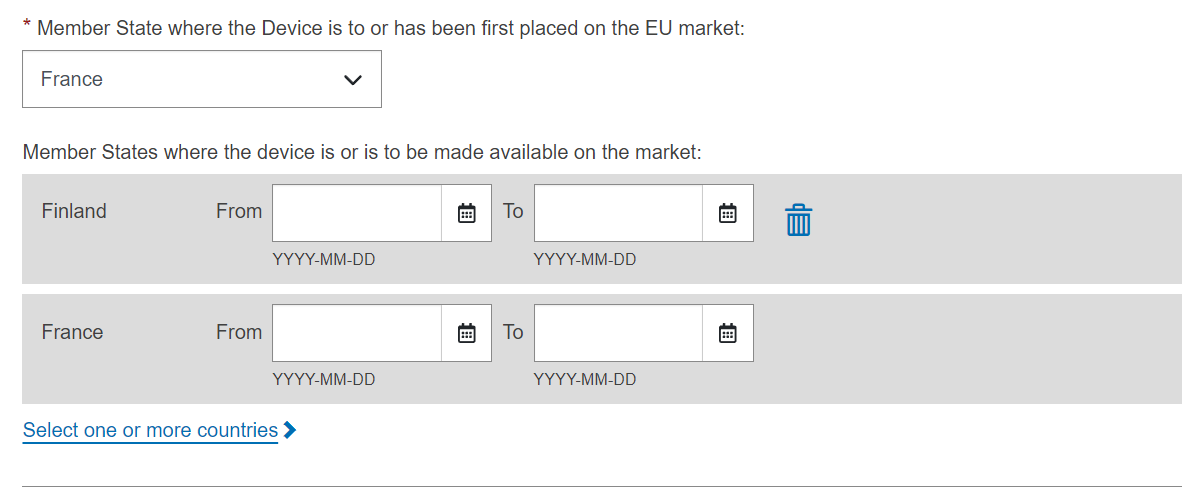
Select a Member State from the drop-down list where the device has been placed on the EU market, and click on Submit to submit it directly or Preview to view before submitting:
A pop-up window will appear asking you to confirm your submission. Once you confirm, you will be brought to a new window confirming the submission of your Legacy device: