Manage your device UDI-DI/EUDAMED ID details
On the dashboard of EUDAMED, click on Manage your Device details:
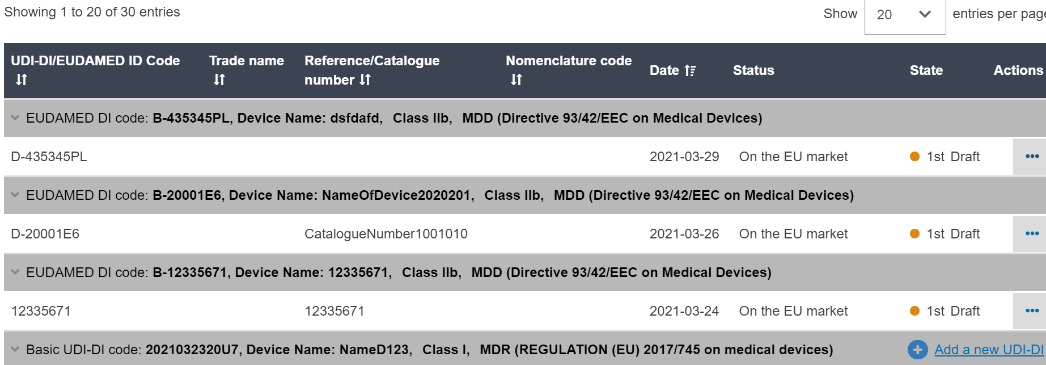
You will see a list:
Note
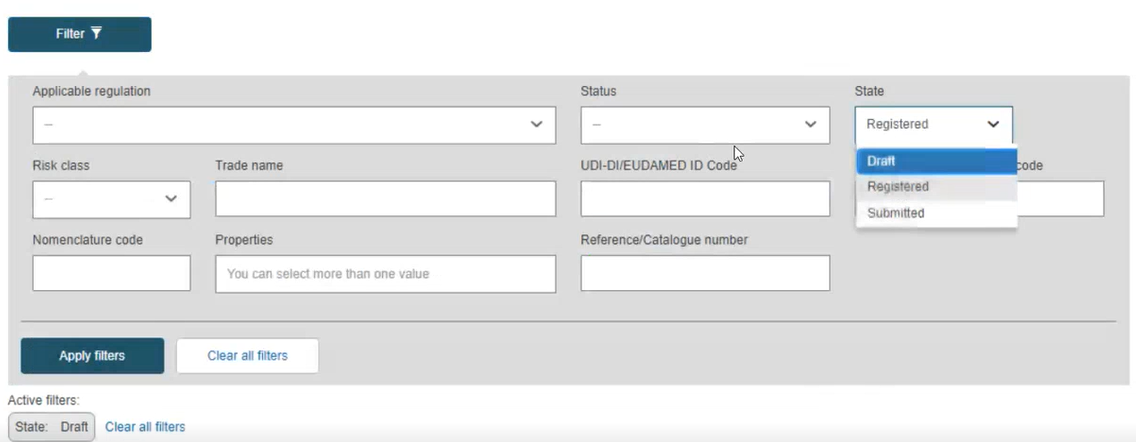
By default, the system lists the devices in draft state. To retrieve other states use the filters:
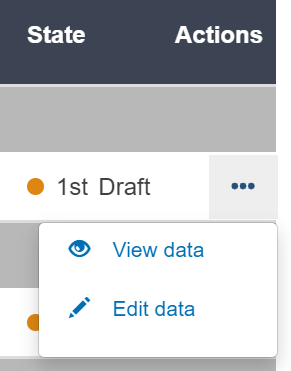
Click on the three dots symbol on the right of the desired entry and then click on View data:
You will see a summary of the details of your device:
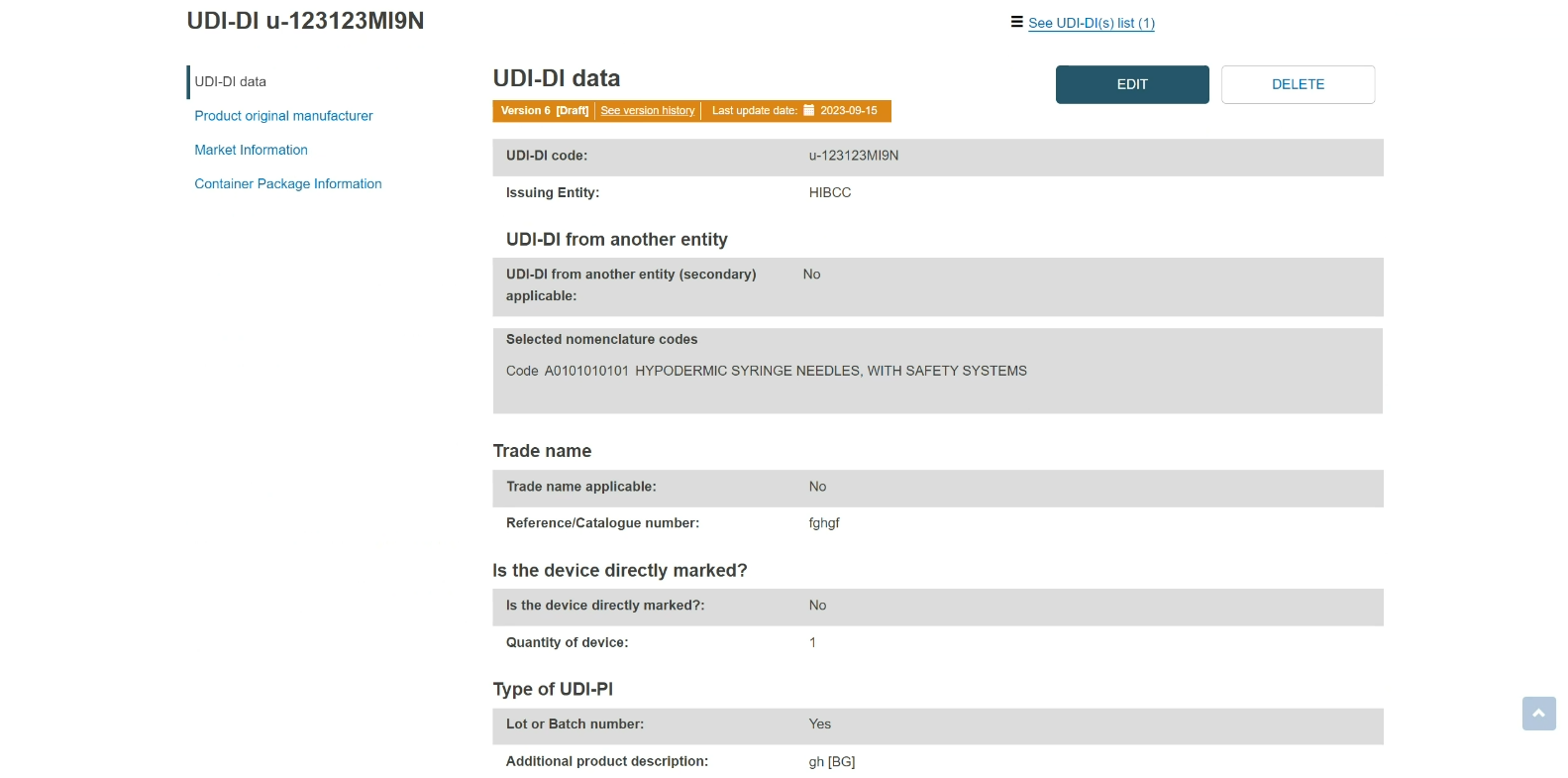
Delete a draft UDI-DI/EUDAMED ID
Update (Create a new version) for UDI-DI/EUDAMED ID
Update (Create new version) for Product original manufacturer
Update (Create new version) for Market Information
Update (Create new version) for Container Packages
Discard registered UDI-DIs/EUDAMED DIs (and their Basic UDI-DI/EUDAMED DI)
Link a registered Regulation Device to a registered Legacy Device
Delete the link between a Regulation Device and a Legacy Device