Categorisation of devices
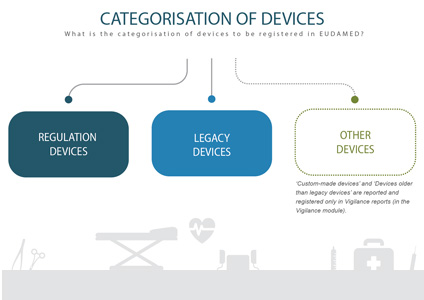
 INFOGRAPHIC: Categorisation of devices
INFOGRAPHIC: Categorisation of devices
Identifiers
The UDI (Unique Device Identification) system is a new feature introduced by the MDR 2017/745 and IVDR 2017/746 EU regulations. It will improve the traceability of medical devices, enhance post-market safety-related activities and allow for better monitoring by Competent Authorities (CA).
BASIC UDI-DI – This is the main access key for device-related information in the EUDAMED database. It is referenced in various other documents [e.g. certificates (including certificate of free sale), EU declaration of conformity, technical documentation and summary of safety and (clinical) performance)]. All devices with the same Basic UDI-DI share the same core characteristics, such as intended purpose, risk class, essential design and manufacturing characteristics. The Basic UDI-DI information entered in EUDAMED includes this core information plus a unique Basic UDI-DI code issued by an officially designated issuing entity. It is independent and different from the packaging/labelling of the device and does not appear on any trade item.
UDI-DI – The UDI is the main identifier of a medical device which is used on its label. It identifies the specific device within a given product family. The UDI-DI is a numeric or alphanumeric code relating to a specific medical device.
(PACKAGE UDI-DI) – If applicable, each device may have an additional, higher-level UDI-DI assigned to its higher package. Package UDI-DIs identify each package format, including quantities of items at each package level.
A Basic UDI-DI always references at least one UDI-DI, while multiple UDI-DIs can be referencing the same Basic UDI-DI.
EUDAMED DI – The EUDAMED DI is equivalent to the Basic UDI-DI. It can either be fully generated by EUDAMED if a UDI-DI has already been assigned to the legacy device, or the DI code can be partly assigned by the manufacturer (EUDAMED is the issuing entity for a EUDAMED DI).
EUDAMED ID – The EUDAMED ID is equivalent to the UDI-DI. In case a UDI-DI has not been assigned yet, the EUDAMED ID will always be automatically and fully generated by EUDAMED from the EUDAMED DI.
A Legacy Device must have an assigned EUDAMED DI (instead of a Basic UDI-DI), and in some cases – when no UDI-DI was already assigned – a EUDAMED ID (instead of the UDI-DI). A legacy device has to be registered in the UDI/Device module of EUDAMED, which allows EUDAMED to process it similarly to a Regulation Device.