Introduction
Economic Operator is defined by MDR and IVDR. The following Economic Operators are Actors who must be registered in EUDAMED:
Manufacturer (MF: EU Manufacturers* & Non-EU Manufacturers**)
Authorised Representative (AR)
System/Procedure Pack Producer (SPPP)
Importer (IM)
* EU manufacturers: If you are established in the EU/EEA, your registration will be assessed by the relevant Competent Authority (CA) of the country where you are established.
** Non-EU manufacturers: If you are not established in the EU/EAA, you need to appoint an Authorised Representative within the Union. Your registration will be verified by the Authorised Representative you indicate in your Actor registration request, before being assessed by the Competent Authority responsible for that AR. The AR you indicate in the registration request must be already registered in EUDAMED.
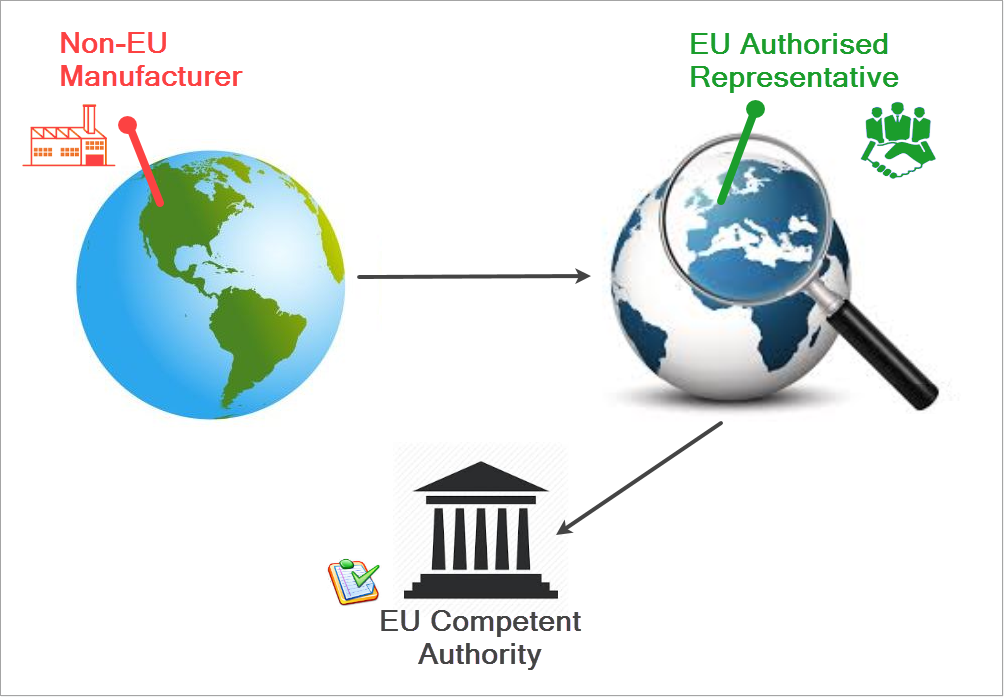 |
The difference in registration process between the two types of MFis highlighted in the Manage section.
Note
Multiple roles
If your organisation has more than one Actor role (e.g. your organisation is a manufacturer and an importer at the same time), you must make a separate actor registrations for each role.