Submission platform for EMDN proposals
What is the purpose of this webpage?
The purpose is to gather input from stakeholders for the annual updates of the European Medical Device Nomenclature (EMDN) in accordance with its key principles, including in particular inclusiveness and public availability. The annual update procedure provides users the opportunity to submit proposals for EMDN terms and codes. Following the assessment of proposals, a new version of the EMDN is released at the end of each calendar year. For more details on the EMDN procedure, kindly refer to the MDCG Guidance titled ‘Procedures for the updates of the European Medical Device Nomenclature’ which can be found here.
Who can participate?
Proposals can be submitted by all actors including but not limited to: competent authorities, notified bodies, the World Health Organization (WHO), trade associations, manufacturers, authorized representatives, importers, distributors, and persons referred to Art.22(1,3).
Timeline
The submission platform is available year-long. The deadline for submitting proposals to be processed in that same calendar year is 31 January 20XX. In 2024, the deadline has been exceptionally extended until 20 March 2024. Proposals submitted after that date are considered and processed in the following calendar year. For more details on the EMDN procedure, kindly refer to the MDCG Guidance titled ‘Procedures for the updates of the European Medical Device Nomenclature’ which can be found here.
How to submit a proposal?
A proposal can be submitted by filling in the online form available below.
Mode of operation
- Enter proposer identification fields (name, surname, contact details, organization/company, type of proposer);
- Select the specific level of the EMDN structure (i.e. select the most granular level, as relevant);
- Select the type of update*;
- Provide details to support the proposal.
All fields in the form are mandatory except for "attachments." Please however note that supporting documents, such as instructions for use, are considered essential documents for the appropriate assessment of a proposal. In order to correctly identify the type of update, please consult the glossary ("Glossary of EMDN update types") available below.
Types of updates
The types of updates possible are as follows:
- Insert a new level (new code and new term)
- Edit an existing term
- Delete an existing level (existing code and existing term)
- Move an existing level (existing code and existing term) to another existing location (category, group or type) in the EMDN
Glossary of EMDN update types:
- Insert a new level (new code and new term): to include device types not currently present in the EMDN (new technologies, request for more detailed of EMDN code/term);
- Edit an existing term: to make an existing term more comprehensive, clearer or to remove any errors. Each modification is aimed at improving usability;
- Delete an existing level (existing code and existing term): in the event that an overlap in codes or critical issues in the structure is to be reported;
- Move an existing level (existing code and existing term) to another existing location (category, group or type) in the EMDN: in case it is not properly placed in the existing hierarchical tree.
Download EMDN (download full list)
1. What is the European Medical Device Nomenclature (EMDN)?
Per Article 26 of Regulation (EU) 2017/745 on medical devices (MDR) and Article 23 of Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR), the European Medical Device Nomenclature (EMDN) aims at supporting the functioning of the European database on medical devices (EUDAMED). Among its various uses, it will be utilised by manufacturers for the registration of medical devices in EUDAMED, where it will be associated to each Unique Device Identifier – Device Identifier (UDI-DI).
As the EMDN primarily serves regulatory purposes to support MDR and IVDR requirements, it also plays a key role in MDR/IVDR device documentation and technical documentation, sampling of technical documentation conducted by notified bodies, post-market surveillance, vigilance and post-market data analysis, etc. It is intended to support all actors in their activities under the MDR/IVDR and provides key device descriptions to patients as regards their own devices and all other devices available on the market and registered in EUDAMED.
2. How was the EMDN created?
According to criteria and requirements set out by the European Commission and EU regulators in the Medical Device Coordination Group (MDCG) and based on orientations provided by the MDCG, the EMDN was founded following a European Commission notice indicating the utilisation of the Italian Ministry’s ‘Classificazione Nazionale Dispositivi medici (CND)’ as the basis for the future EMDN.
At that time, the CND was already utilised in three Member States (Italy, Greece and Portugal) and supported the registrations of a variety of EU and international manufacturers within the EU.
During the course of 2019 and 2020, consultations and preparatory work on the CND took place with stakeholders and key experts. A first version of the EMDN was released on 4 May 2021.
3. What are the key principles of EMDN?
The EMDN is based on fundamental key principles jointly set out by the European Commission and EU regulators. These principles include but are not limited to:
- a. Regulators-led: regulators play a key role in managing, validating, updating and advising on the nomenclature.
- b. Structured: the nomenclature has transparent hierarchies by which terms and codes could be meaningfully clustered into groups and types.
- c. Predictable: the structure and content remains sufficiently stable to allow various regulatory uses of the nomenclature, in a manner which still allows for the accommodation of technological innovation.
- d. Transparent: the policies for updates of the nomenclature terms and descriptions are sound and reflect the needs of regulators and the wider healthcare community.
- e. Inclusive: the periodic reviews are open to all, based on real-world use and demonstrable needs.
- f. Available: the terms, descriptions and codes are available, in full, to all users.
- g. Accessible: no manufacturer or natural/legal person should be subject to fee or suffer from any discrimination, compared to other operators, in relation to the use of the nomenclature.
- h. International: internationally recognised at the time of the date of application of the MDR/IVDR.
4. How do I gain access to the EMDN?
The entirety of the EMDN is accessible to all stakeholders, free of charge. It can hence be utilised by a non-exhaustive list of stakeholders such as manufacturers, patients, research organisations, practitioners, hospitals, pharmacies etc. The EMDN can be accessed and downloaded in pdf and excel format through this page and the European Commission’s website page for MDCG documents.
5. How is the EMDN structured?
The EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree. It clusters medical devices into three main levels:
- Categories: the first hierarchical level,
- Groups: the second hierarchical level,
- Types: the third hierarchical level (which expands into several levels of detail (1°, 2°, 3°, 4° and 5°), where necessary.
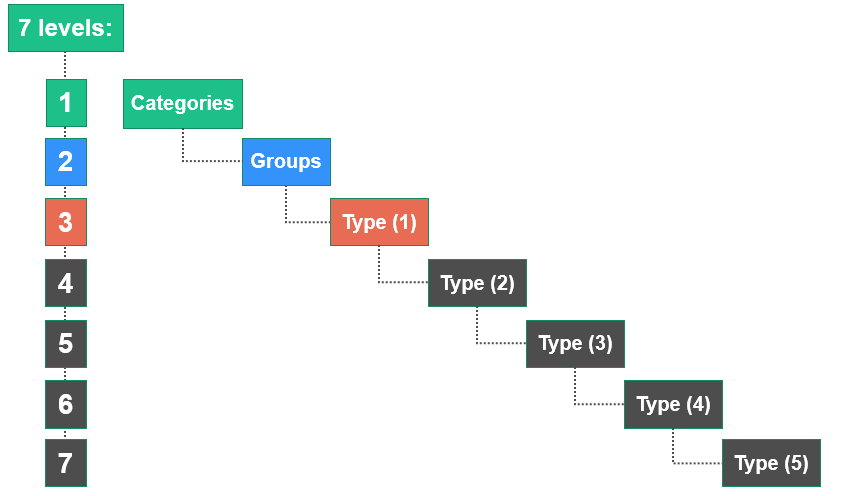
Each alphanumeric code begins with a letter referring to the ‘CATEGORY’ for which the device falls under, followed by two numbers indicating the ‘GROUP’ and a series of numbers which refer to the ‘TYPE’. The maximum number of digits is set at 13.

6. Which level of the EMDN should I use to assign a term to my device?
Using the tree-like hierarchy of EMDN, users must always assign the most granular and terminal term available (lowest level in the tree) to their device.
